Introduction
The ability to explore the outside world
and compare old and new information is critical for animal's survival. The
proper exploratory behavior in novel unpredictable situations (brought about by
weather changes, activity of other animals, etc.) allows distinguishing
meaningful and ignoring not important novel stimuli and their combinations.
Habituation, one of the simplest forms of non-associative learning, is the
mechanism providing an animal with the means to dampen the perception of repetitive neutral stimuli and
be ready to effectively detect a novel stimulus with a yet unknown significance,
and therefore is vitally important for the interaction of an organism with its
environment [1-3]. Both response to novelty and habituation change with age,
but molecular mechanisms underlying these changes remain mostly unknown.
Recently we have demonstrated that a deficiency of the
transcription factor BMAL1 in mice results in accelerated aging [4]. BMAL1
activity is critical for the operation of the circadian clock - a genetically
determined time-keeping system generating 24-hour oscillations in physiology
and behavior known as the circadian rhythms [5]. The involvement of the
circadian clock in the control of brain-based activities such as sleep [6],
reward behavior [7,8] and regulation of mood [9,10] has been reported.
Recently, a connection between the circadian clock and memory has been
suggested: mice with deficiencies of different components of the circadian
clock demonstrate impairments of some types of memory and learning [11]. Here
we hypothesize that the circadian clock is involved in the regulation of the
adaptation to the new environment, and investigate this hypothesis using a set
of circadian mutants - mice with targeted disruptions of circadian genes Bmal1
or Cry1 and Cry2, or with the mutation of the Clock
[12-14] gene. These genes encode proteins representing the core components of
the circadian clock. Transcription factors BMAL1 and CLOCK form a transcription
complex activating expression of target genes including circadian transcription
repressors CRY1 and CRY2. In turn, CRY1 and CRY2 suppress activity of the
BMAL1:CLOCK complex, including their own expression, thus generating a negative
feedback loop; expression of several other genes important for the functional
clock (i.e. Per1, 2 and 3) is also under the transcription control of
the BMAL1:CLOCK complex [15].
Results
Hyperactivity
and impaired habituation of Bmal1-/- mice
The exploratory behavior of the wild type
and Bmal1-/- mice in novel environment was tested in the open field
paradigm (OF). For this, 3-months old male mice were placed in a bright-lit
50x50 inches square box and monitored for the pattern of their exploratory
behavior for 1 hour with 5-min resolution. In order to assess intersessional
habituation, animals were exposed to the same environment 24 hours later.
Activity of Bmal1-/- mice in novel environment was strikingly different
from that of wild type mice. Bmal1-/- mice demonstrated significantly
increased locomotion (horizontal activity) on day1 [F[1,5] = 19.21, P = 0.007]
and day2 [F[1,5] = 27.36, P = 0.0004] (Figure 1, left panel). Total distance
traveled by Bmal1-/- animals during 1h on days 1 and 2 of the OF
experiment was respectively 2.7-fold and 4.7-fold higher than that of the wild
type mice. The pattern of activity in wild type and Bmal1-/- mice was
also very different. As expected based on previous studies [1,16], on day1 the
distance traveled by wild type mice during the first 15 min of the OF session
was 3.1-fold higher than during the last 15 min [t-test P <0.0001]; on day2,
it was 2.8-fold higher [t-test P <0.001]. Such a decrease with time on both
first and second days of the experiment results from animals' habituation to a
new environment within each OF session. When compared to day1, total activity
of wild type mice on day2 was also significantly reduced [F[1,5] = 13.37, P =
0.015] (Figure 1 and 5, left), indicating that a long-term memory reflecting
experience obtained on day1 has been formed [17]. In contrast, on day1 the
difference in distance traveled by Bmal1-/- mice during the first 15 min
of the OF session was only 1.6-fold higher than during the last 15 min [t-test
P <0.01], whereas there was no difference on day2. Remarkably, the total
distances traveled by Bmal1-/- mice during the first and the second days
were virtually identical (Figure 5, lower left), and no statistically
significant difference was detected between days 1 and 2 of the test [F[1,5] =
3.83, P = 0.107] (Figure1, left).
In
the same experiments, vertical (rearing) activity was measured by registering
the sequential crossing of beams up and down in vertical direction. Similar to
the difference in horizontal activity, total rearing activity of Bmal1-/-
mice was significantly increased compared to wild type animals (2.3-fold and
4.7-fold on days 1 [F[1,5] = 13.92, P = 0.014] and 2 [F[1,5] = 22.57, P =
0.00031], respectively (Figure 1, right panel). On day2 wild type mice
demonstrated 1.9-fold reduction in total rearing activity [F[1,5] = 24.34, P =
0.004], whereas no difference between the two days was detected in Bmal1-/-
animals [F[1,5] = 0.37, P = 0.568] (Figure 5, lower right). In contrast to
differences displayed in horizontal activity, animals of both genotypes
displayed similar rearing activity during the first 5 min of exposure to the
new environment. After this, rearing activity of wild type animals gradually
decreased, whereas in Bmal1-/- mice it increased and stayed elevated for
the duration of testing (Figure 1, right). On day 2, the temporal pattern of
rearing activity in animals of both genotypes did not differ much from the one
displayed on day1. Taken together, these data indicate that deficiency in core
circadian component, BMAL1, affects not only rhythmicity in locomotor activity,
but other patterns of behavior as well. Specifically, when placed in a new
environment, BMAL1-deficient mice display novelty-induced hyperactivity in
locomotion and rearing behavior, and deficits in inter- and intrasessional
habituation.
BMAL1
is expressed in hippocampal and cortical neurons
BMAL1
is expressed in many different tissues and organs. Brain-specific Bmal1
expression at the mRNA level has been demonstrated for the suprachiasmatic
nucleus of the anterior hypothalamus (SCN), the residence of the master
circadian clock, as well as for other brain regions including the cortex and
hippocampus formation [12,18]. To investigate the distribution of BMAL1
protein in the brain, we performed in situ immunofluorescent staining
using BMAL1-specific antibody (Figure 2a). High expression of
BMAL1 was detected in the pyramidal neurons of the hippocampus and in the
neurons of the subiculum and enthorhinal cortex of wt mice. Neurons of the
neocortex were also positive for BMAL1. Specificity of the signal was confirmed
by the parallel staining of the brains of Bmal1-/- mice. Thus, BMAL1
protein is expressed in brain structures associated with memory formation.
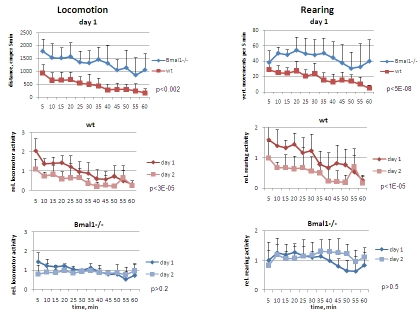
Figure 1. Open field analysis of exploratory activity and habituation of wild type and Bmal1-/- mice.
Locomotor and rearing activity were measured in 5 min increments during 1
hr. (Upper panel) locomotor and rearing activity of wt and Bmal1-/-
mice on day1; (middle panel) relative locomotor activity (normalized to
average distance covered on day1) and relative rearing activity (normalized
to average rearing level on day1) of wt mice on days 1 and 2; (lower panel)
relative locomotor and rearing activity of Bmal1-/- mice on days 1
and 2 (* P<0.05).
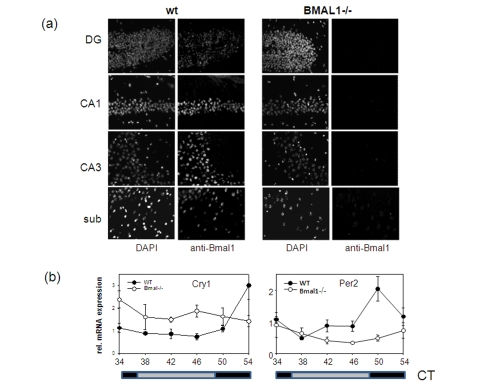
Figure 2. Expression of circadian proteins in brain structures. (a)
Immunostaining of sagittal brain sections of wt and Bmal-/- mice
with BMAL1 specific antibodies. Counterstaining with DAPI was used to
detect nuclei. Pyramidal neurons of hippocampal areas CA1 and CA3, granular
cells of the dentate gyrus and neurons of subiculum expressing BMAL1 are
shown. (b) Circadian profile of Cry1 and Per2 mRNAs in
the brain of wt (filled circles) and Bmal1-/- mice (open circles) as
measured by real-time PCR. (* p<0.05).
BMAL1
deficiency disrupts circadian expression of mPer2 and mCry1 genes
in the brain
In
complex with CLOCK (or its close tissue-specific homolog, NPAS2) BMAL1 controls
rhythmic expression of target genes, and BMAL1 deficiency results in disruption
of rhythmic pattern of gene expression in the SCN and liver [12]. We decided to
investigate how the absence of BMAL1 will affect the expression of BMAL1 target
genes in the brain. As demonstrated in Figure 2b, rhythmic pattern of
expression of two core circadian genes, mPer2 and mCry1 in the
brain of BMAL1-deficient animals was significantly impaired with the mPer2
gene being mostly down-regulated and mCry1 up-regulated. This pattern of
expression was previously observed in the SCN and liver of Bmal1-/- mice
and was attributed to the dual role of the BMAL1:CLOCK complex in transcription
regulation [19]. Importantly, both PER2 and
CRY1 were recently implicated in memory and learning [20,21].
BMAL1
deficiency results in disruption of ROS homeostasis in the brain
Aging is associated with increased oxidative stress in
many tissues, including the brain. Recently, oxidative stress and misbalance in
reactive oxygen/nitrogen species (ROS/RNS) homeostasis was proposed as a
mechanism for age-dependent changes in brain physiology, including decline in
memory and learning [22]. Previously we have demonstrated that BMAL1 is
directly involved in the regulation of ROS/RNS homeostasis, and that
accelerated aging characteristic for Bmal1-/- mice, at least in part,
can be attributed to excessive production of ROS in some tissues of Bmal1-/-
animals [4]. This prompted us to compare the levels of ROS in the brain of wild
type and Bmal1-/- mice.
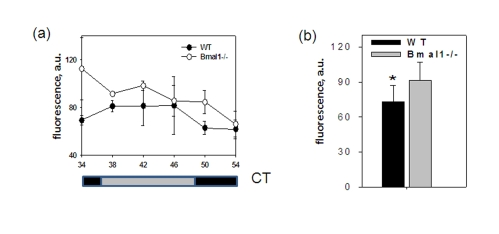
Figure 3. BMAL1 deficiency disrupts ROS homeostasis in the brain. (a) ROS
level in the brain of wt and Bmal1-/- mice was detected in the indicated
time points of the circadian cycle. (b) Average ROS level in the
brain of wt and Bmal1-/- mice for 24h (* P<0.05).
To
account for possible daily fluctuations, the level of ROS was measured in
brains of wild type and Bmal1-/- mice collected throughout the day every
4 hrs. The level of ROS in the total brain extracts did not show any obvious
circadian pattern; however, at each time point it was significantly higher in Bmal1-/-
mice (except for the time of maximum level of ROS for the wild type (CT34)). As
a result, in mutant mice the average daily levels of ROS in the brain were 20%
higher [t-test P < 0.01] (Figure3b). Thus, BMAL1 deficiency results in
excessive production of ROS and chronic oxidative stress in the brain, which
may affect various brain-specific metabolic processes including memory
formation.
Deficiency
of circadian proteins CLOCK and CRY1,2 differentially alters habituation and
exploratory activity
The
observed behavioral phenotype of Bmal1-/- mice may result from
desynchronization of physiological activity of neurons due to disruption of the
circadian oscillator. On the other hand, it may be unique to Bmal1-/-
mice and result from disruption of the BMAL1-dependent control of tissue
homeostasis. To discriminate between these two possible mechanisms in the
regulation of hyperactivity and habituation, we studied the exploratory
behavior of arrhythmic mice with disrupted activity of other circadian
proteins.
We have chosen mice with the deficiency
of the two Cry genes (Cry1,2-/- double knockout mice) and mice with the homozygous mutation of the BMAL1 transcription
partner, CLOCK (Clock/Clock mutant mice). Previous work has shown that
these two models, along with the Bmal1-/- model, may be approximated by
two opposite functional states of the BMAL1:CLOCK transcription complex. Thus,
functional deficiency in BMAL1 or CLOCK proteins results in the absence of
transactivation of the target genes, while the absence of the CRY1,2 proteins
cause constantly elevated expression of circadian target genes. All these
mutants demonstrate disruption of rhythmic pattern of locomotor activity and at
the gene expression level [12-14].
Cry1,2-/- and Clock/Clock mice were placed in novel
environment, similarly to experiments described for wild type and Bmal1-/-
animals. In contrast to Bmal1-/- mice, they did not demonstrate
hyperactivity in locomotion: the activity of Clock/Clock on day1 was
indistinguishable from that of the wild type [F[1,5] = 1.77, P = 0.241], while Cry1,2-/-
animals were even less active [F[1,5] = 3.23, P = 0.132] (Figure 4 and 5, left
panels). Both Clock/Clock [F(11,55) = 13.12, P<0.001] and Cry1,2-/-
[F(11,55) = 8.02, P<0.001] mice showed intrasessional habituation on day1
similar to that of the wild type mice: distance traveled during the last 15
minutes decreased more than 2 fold compared to the first 15 minutes. On day2, Clock/Clock
mice demons-trated the level of locomotor activity indistinguishable [F[1,5] =
3.95, P = 0.103] from that on day1. Thus, although Clock/Clock mice
showed intrasessional habituation similar to wild type (both on days 1 and 2),
there was no intersessional habituation (no significant difference between days
1 and 2). These data suggest that that Clock/Clock mutant mice have
normal formation of the immediate memory of novel context and impaired
long-term memory. Locomotor activity of Cry1,2-/- mice on day2 was
significantly lower than on day1 (Figures 4 and 5, left) (2.0 folds, [F[1,5] =
27.19, P = 0.003]), suggesting that Cry1,2-/- demonstrate both intra-
and intersessional habituation.
While the level of horizontal activity of Clock/Clock
mutants was similar to the horizontal activity of wild type mice, Clock/Clock
mutants demonstrated elevated rearing activity [F[1,5] = 7.65, P = 0.04],
which was intermediate between that of wild type and Bmal1-/- animals (Figure 4 and 5, right). In contrast with the case of locomotion (normal intarsessional
habituation and no intersessional habituation), there was no difference in rearing activity of Clock/Clock mutants between
day1 and day2 [F[1,5] = 0.44, P = 0.538], and only insignifi-cant decrease in
rearing between the first and the last 15 min of the experiment on both days
(T-test P = 0.1 and P = 0.6, respectively). Rearing behavior of Cry1,2-/-
mice on day1 was similar to wt (with a tendency to be lower) (T-test=0.6) (Figure 5, right); however, rearing activity of Cry1,2-/- on day2 constantly
remained at the habituated level and was significantly lower than wt [F[1,5] =
48.63, P<0.001] (Figure 4, right).
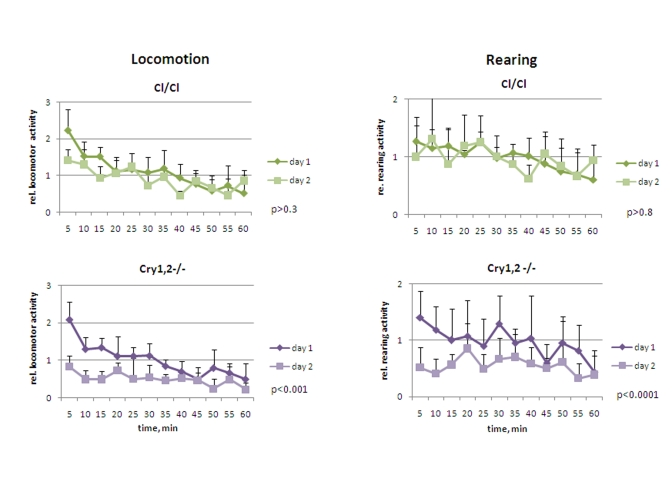
Figure 4. Open field analysis of exploratory activity and habituation of Clock/Clock and Cry1,2-/- mice. Relative locomotor and rearing
activity of Clock/Clock and Cry1,2-/- mice on days 1 and 2
(normalized to the average distance/activity level on day1) (* P<0.05).
Taken together, these results demonstrate a correlation
between the level of activity and memory formation on one hand and transcription
status of the BMAL1:CLOCK complex on the other.
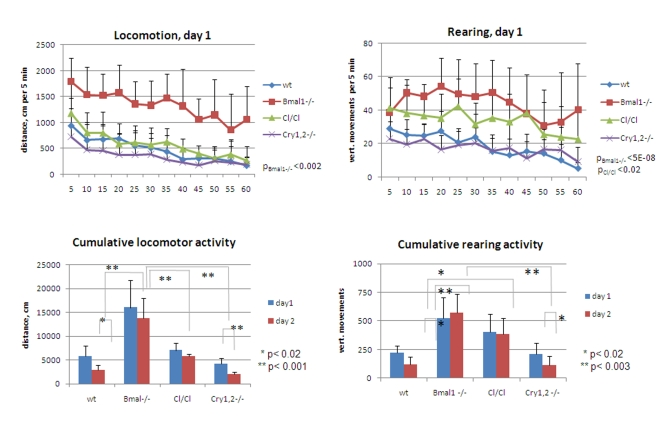
Figure 5. Exploratory
activity of wild type and circadian mutant mice. (Upper panel)
Locomotor and rearing activity measured in 5 min increments during 1 hr on
day1 for wt (diamonds), Bmal1-/- (squares), Clock/Clock
(triangles) and Cry1,2-/- (crosses) mice. Statistically significant
difference with wt activity is shown as p values. Difference between wt, Clock/Clock
and Cry1,2-/- locomotor activities, and between rearing activities
of Bmal1-/- vs. Clock/Clock and wt vs. Cry1,2-/- is
not statistically significant. (Lower panel) Cumulative traveled
distance and rearing activity on days 1 and 2; For upper panels p values of
statistically significant differences between wild type and circadian
mutants are indicated. For lower panels * P<0.05.
Deficiency
in activity of the core circadian proteins BMAL1, CLOCK or CRY1,2 results in
different behavioral patterns in the open field
Cumulative
data on locomotor and rearing activities showing significant differences
between the animals of all tested genotypes are summarized in Figure 5. Thus,
total distance traveled by Bmal1-/- mice on both days greatly exceeded
the distance traveled by the wild type [day1 fold 2.8, t-test P <0.01; day2
fold 4.7, P <0.001], Clock/Clock [day1 fold 2.3, t-test P <0.01;
day2 fold 2.4, P <0.01], or Cry1,2-/- [day1 fold 3.9, t-test P
<0.001; day2 fold 6.9, P <0.001] animals (Figure5, left panels). Cry1,2-/-
mice demonstrated the lowest level of horizontal activity, while the locomotion
of the Clock/ Clock animals was comparable to that of the wild type. Remarkably, the horizontal activity of Bmal1-/-
and Clock/Clock mutants remained the same on both days of the experiment, whereas wild type and Cry1,2-/-
animals demonstrated significant reduction in activity on day2 [wt fold 2.0
t-test P <0.02; Cry1,2-/- fold 2.1, P <0.01] (Figure 5, lower
left). Rearing activity of Bmal1-/- and Clock/Clock animals was
elevated compared to wt [Bmal1-/- day1 fold 2.3, t-test P <0.01, day2
fold 4.7, P <0.001; Clock/Clock day1 fold 1.8, P <0.01, day2 fold
3.2, P <0.001] and showed no differences between the two sessions. In
contrast, wild type and Cry1,2-/- mice showed low rearing activity and
robust intra- and intersessional habituation (Figure 5, right panels).
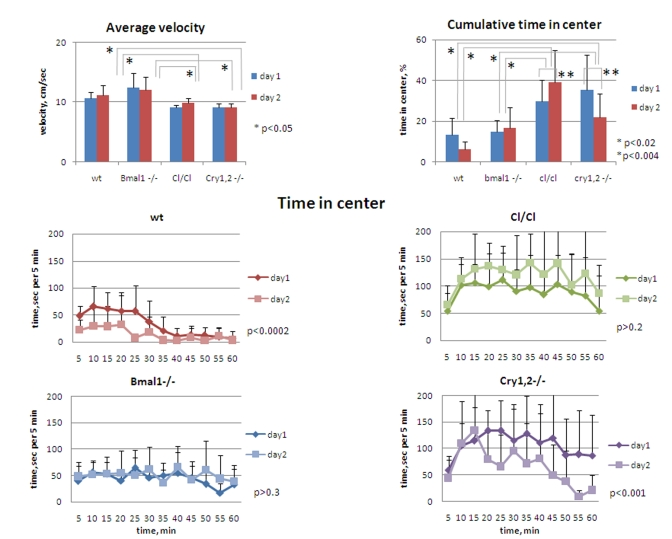
Figure 6. Circadian
mutant mice do not demonstrate anxiety phenotype. (Upper panel,
left) Average horizontal velocity for all genotypes measured during 1h
on days 1 and 2. Average velocity does not significantly differ between wt
and Bmal1-/- animals; slight (~15%) but statistically significant
decrease in average velocity is detected for Clock/Clock and Cry1,2-/-
mice compared with wt and Bmal1-/-. (Upper panel, right)
Cumulative time spent in the center for all genotypes measured during 1 hr
on days 1 and 2. (Lower panel) Time spent in the center square of
the open field arena on days 1 and 2 by wt, Clock/Clock, Bmal1-/-,
and Cry1,2-/- mice. *P<0.05.
Circadian
mutants demonstrate normal or decreased anxiety levels in the open field
The increase/decrease in the distance
traveled by different circadian mutants may result from either differences in
time spent in motion vs. rest time, or from the differences in the velocity
among the genotypes. However, an average velocity of animals, calculated based
on horizontal distance and time in horizontal motion, was mostly uniform in
animals of all genotypes, except for Clock and Cry1,2-/- mice
that showed slight (~15%) but statistically significant reduction of speed
[t-test P <0.05] (Figure 6, upper left). This suggests that Bmal1-/-
and Clock/Clock mice were in fact longer in motion,
whereas Cry1,2-/- were less in motion on both days when compared to wild
type, rendering a hyperactivity phenotype for Bmal1-/- and Clock/Clockanimals.
High
locomotor and rearing activity and deficit of contextual habituation often
correlate with elevated level of anxiety, which can be accessed by the amount
of time spent in the center of the OF (a risk-taking behavior) [23]. To
evaluate the level of anxiety in animals of all four circadian genotypes, we
compared the time they spent in the central zone of the OF (Figure 6). On day1
of the experiment, Bmal1-/- and wild type mice spent about 14% of the
time in the central zone, while the corresponding time in Cry1,2-/- and Clock/Clock
mice was more than two-fold higher (37% and 30% respectively [t-test P
<0.01]) (Figure 6, upper right). The differences between the wild type and Bmal1-/-,
and between Clock/Clock and Cry1,2-/- animals were not
significant. Compared to day1, the amount of time spent in the center on day2
was not changed in Bmal1-/- , whereas in wild type and Cry1,2-/-
mice it was decreased two-fold [t-test P <0.01]. Interestingly, cumulative center
time of Clock/Clock mice on day2 showed even a tendency for increase but
did not reach statistical significance. Thus, none of the tested circadian
mutants displayed a pro-anxiety phenotype in the open field paradigm. On the
contrary, Clock/Clock and Cry1,2-/- demonstrated opposite, more
risk-taking behavior. A decrease in center time on day2 observed in wild type
and Cry1,2-/- mice correlates with a decrease in total locomotor and
vertical exploratory activity (indicative of habituation). Bmal1-/- again
did not demonstrate any difference in performance between days 1 and 2, while Clock/Clock
mutant mice demonstrated an increase in time spent in the center.
Interestingly, mice of different genotypes had different patterns of the
risk-taking behavior. Wild type mice "took the risk" of short raids in the
middle of the brightly-lit arena during the first 30 min and then moved mainly
along the walls or sit in one of the corners (Figure 6, middle left). Bmal1-/-
mice continued to move across the center during the entire session (Figure 6,
lower left). Both Cry1,2-/- and Clock/Clock mice had an increase
in time spent in the center after first 10 min, during which they had prolonged
periods of sitting in the middle of the arena - a very unlikely behavior for wt
mice (Figure 6, middle and lower right). These data clearly demonstrate the
lack of correlation between the locomotor/rearing activity and time spent in
the center of the open field in different circadian mutants. Thus, hyperactive Bmal1-/-
demonstrated "normal" level of anxiety, while both hyperactive Clock/Clock
and hypoactive Cry1,2-/- had decreased level of anxiety. Therefore,
hyperactivity and deficit of contextual habituation of Bmal1-/- and Clock/Clock
cannot be explained by the increase in the level of anxiety in these mice.
Discussion
Decline in mental performance, including
deficits in memory formation, learning and adaptation to novelty are hallmarks
of aging. At the same time, it is well documented that the activity of the
circadian clock decreases with age [24]. Reciprocal relationships between the
decline in the circadian clock activity and deterioration in mental performance
are currently a subject for active discussions [25]. In this study we propose
that the activity of the circadian proteins is important for adaptation to
novelty, which is one of the aspects of daily interactions between an organism
and its changing environment. Our results demonstrate that habituation to
novelty is differentially altered in mice with a deficiency/mutation of the
core circadian genes Bmal1, Clock, or Cry1 and Cry2 and
correlates with the transcription activity status of the BMAL1:CLOCK: [CRY1,2] complex.
Exploration behavior is thought to be induced by a novelty
detected by the hippocampus which works as a comparator of the stored spatial
"maps" - memory of visited places - and perception of an unknown space. As the
animal acquires information of the new space, a novel spatial map is generated
and exploratory behavior ceases (which is referred to as intrasession
habituation in the open field paradigm, and depends on working memory). When
placed in the same environment on consequent days, mice demonstrate
significantly reduced exploratory activity, which is interpreted as a sign of
acquiring of long-term memory about the place (intersessional habituation)
[1-3,17]. Thus, habituation is thought to depend on short- and long-term
memory [26-28]. Deficiency in the intrasession habituation of Bmal1-/-
mice is indicative for working memory impairments. Recently, circadian
modulation of short-term memory was shown in Drosophila [29] and humans [30]. Severe
deficiency in the intersession habituation demonstrated by Bmal1-/- and Clock/Clock
mice both in locomotion and rearing suggests that Bmal1-/- and Clock/Clock
mice lack memory of the previous day experience and allows speculating that
transcription activity of the BMAL1:CLOCK complex is necessary for the LTM
formation, which requires de novo synthesis of both RNA and protein
[31]. Importantly, LTM was shown to depend on time of the day for LTM
acquisition/retrieval [32], which may reflect daily fluctuations in BMAL1:CLOCK
transcription activity. Facilitation of both intra- and intersession
habituation demonstrated by Cry1,2-/- mice further strengthens the role
of BMAL1, CLOCK and CRY1,2-associated transactivation and transrepression of
gene expression in memory function. Interestingly, although Cry1,2-/-
mice exhibit a deficit in time-place learning, which was attributed to
disrupted time-keeping system, no deficits were observed in learning abilities
of Cry1,2-/- mice in several not-time associated learning tasks [21].
Our results suggest that LTM and/or STM formation in these animals is
facilitated; however, more specific learning/memory tests are necessary to
dissect various types of memory influenced by CRY1,2 as well as other circadian
proteins. These observations and several recent reports indicate a close
connection between the activity of the circadian system and memory formation.
Circadian cycling was recently proposed as a mechanism for the proper memory
consolidation [33], probably through the circadian oscillation of MAP kinase
activity reported in the mouse hippocampus [34]. Circadian modulation of memory
formation has been shown for different model organisms such as Aplysia,
Drosophila, zebrafish and rodents; a growing body of evidence implicates the
circadian regulation of learning and cognitive performance in humans [11]. More
data on specific roles of individual circadian proteins in different forms of
memory are accumulated from studies of mice with deficiencies in these
proteins. Thus, mice deficient in NPAS2 have
impaired cued and contextual fear memory [35]. Per2-/- mice demonstrate
impairment in trace fear memory, but not in cued fear memory [20]. Per1-/-
mice exhibit spatial learning deficits in the radial arm maze [18].
Exposure to a novel environment is mildly stressful and
inherently arousing experience for mice [1-3]. Failure of the Bmal1-/- mice
to habituate within a single session could also be attributed to their
inability to cope with the novelty-induced stress resulting from functional
disruption of one or several brain modulatory systems [2], which might be the
cause of hyperactivity in both locomotion and rearing in these mice. Indeed,
the circadian clock is involved in control of the rate-limiting enzyme in the
biosynthesis of dopamine - tyrosine hydrolase (TH, also known as monooxygenase)
expression; TH expression is reduced in Per1-deficient mice [9], whereas
it is greatly elevated in Clock/Clock mutants [8]. Significant
hyperactivity in the open field was reported for several transgenic mice with
disturbed dopamine regulation [36]. Interestingly, recently was shown that
modulation of the hippocampus-dependent memory by attention is
dopamine-mediated [37]. Further study on dopamine level and bioavailability in
circadian mutants will help to determine whether the observed changes in the
activity of circadian mutants occur through dopamine-dependent or independent
mechanisms. On the other hand, hyperactivity is often associated with elevated
anxiety [38]; however, the anxiety level of Bmal1-/- mice did not
significantly differ from the wild animals judging by the time spent in the
center of the arena. Importantly, hyperactivity of Bmal1-/- mice was
associated with novelty, because average home cage activity does not differ
between wt and Bmal1-/- animals [12]. In contrast, Clock/Clock
mutant mice exhibited the pattern of horizontal activity similar to wild type;
however, their rearing activity was almost two-fold higher compared with wt.
Horizontal locomotion correlates with cognitive component of exploratory
behavior, while rearing behavior is considered to reflect motivational
component [28]; together with the fact that Clock/Clock mutants spent in
the center of the open field arena twice more time than wt animals, these data
suggest that Clock/Clock mice demonstrate enhanced activity-based
arousal and reduced anxiety, which is in good agreement with observations made
by [39]. In sharp contrast to Bmal1-/- and Clock/Clock mutant
mice, Cry1,2-/- mice were less active in the open field experiments; at
the same time, time spent in the center of the open field arena was almost
three-fold higher in Cry1,2-/- mice when compared to wild type and Bmal1-/-,
and was comparable with that of the Clock/Clock mutants - the pattern
which can be interpreted as a sign of greatly reduced anxiety in these animals.
These observations reinforce the previously reported data on the involvement of
the circadian proteins in the regulation of mood [8,9].
ROS/RNS are important regulators of cellular signaling; any
misbalance can be critical for brain physiology and affect various mental
functions, [40-42], therefore, their production and detoxification are tightly
controlled by the system of ROS/RNS-generating and antioxidant enzymes. Chronic
oxidative stress in an aging brain is one of the main reasons for
age-associated mental decline [22]. Here we show that BMAL1 deficiency
significantly disturbs the normal ROS level in the brain. Thus, BMAL1-dependent
control of ROS can be one of the potential mechanisms of the observed
behavioral changes of the circadian mutants. Indeed, as already has been
mentioned above, the circadian oscillation of MAP kinase activity in the
hippocampus is critical for memory formation, although the mechanisms of cyclic
activity of MAPK are unclear [34]. ROS are critical regulator of MAP kinase
activation and MAP kinase signaling pathway [43], thus, the observed circadian
oscillation of ROS level in the brain can be at least partially responsible for
the oscillation of MAPK activity.
Using
experimental settings within the open field paradigm, which embraces the
established behavioral tests for exploration and adaptation to novelty in rodents,
we have found that activity of the core circadian clock proteins BMAL1, CLOCK
and CRY1,2 is necessary for the regulation of exploratory behavior in mice.
Opposite phenotypes of Bmal1-/- and Clock/Clock mutant mice on
one hand and Cry1,2-/- on the other suggest that the changes in the
novelty-induced behavior in these animals are not the result of the general
disruption of the circadian clock, but rather indicate that individual protein components of the molecular clock
play non-identical roles in habituation. Therefore, the exploratory performance
depends on the mutual balance of activities of these proteins, while the
general regulation of these activities by the circadian clock warrants the
optimization of the performance. It is well documented that aging affects the
circadian system [24]; here we suggest that aging also affects the mutual
balance between circadian proteins, which in turn affects mental performance.
Our results suggest the involvement of the circadian proteins in
fundamental processes of memory formation, and
encourage further investigations into the role of the circadian proteins in
memory, learning behavior and age-associated mental decline.
Experimental
procedures
Animals.
Bmal1-/-mice were obtained from Dr. C.
Bradfield (University of Wisconsin) [12], Clock mutant mice were
obtained from Dr. J. Takahashi (Northwestern University) [13], and Cry1,2-/-knockout mice were obtained from Dr. A. Sancar (University of North
Carolina at Chapel Hill) [14]; details of target gene knockout strategies and
animal generations can be found in the above cited publications. All mutants
were backcrossed to C57BL/6J inbred strain (The Jackson Laboratory, Bar Harbor, ME, USA) for 12 generations. Wild type and Bmal1-/- mice were
generated by breeding of Bmal1+/- males with Bmal1+/- females. Clock
mutants were generated by breeding of Clock/Clock males with Clock/wt
females, Cry1,2-/- were generated by breeding of Cry1,2-/- males
with Cry1+/-, Cry2-/- females. Wild type mice generated as a result of Bmal1+/-
breeding were used as a control for all experiments (since after 10 backcross
generations the line is 99% genetically identical to the recipient strain,
mutants (backcrossed to C57BL/6J for 12 generations) and wild type were
considered as congenic with C57BL/6J background). For all experiments wild
type and mutant mice were randomly picked from several independent litters.
Animals were maintained on a 12:12 light:dark cycle with lights on at 7:00 am,
on regular diet. For tissue collection animals were transferred to constant
darkness and tissue samples were collected with 4 hour intervals beginning
after 34 hours of exposure to DD, immediately frozen on dry ice and stored at -
80°C. All animal studies were conducted in accordance with the regulations of
the Committee on Animal Care and Use at Cleveland State University and Roswell
Park Cancer Institute.
Open field exploration.
A mouse was
placed in the bright-lit 50x50 inches Plexiglas square box, and the activity of
the animal was monitored with 16x16 photobeam activity system (San Diego
Instruments). Animal activity was recorded every 5 minutes during 1 hour on the
day1 and day2 (24h later), and analyzed using Open Field Software. All
experiments were performed with 3-4 month old male mice between 11 am and 4 pm,
at least 6 animals of each genotype were analyzed.
RNA isolation and real-time PCR analysis.
Total RNA was isolated from the brain with TriZol
reagent (Invitrogen) according to the manufacturer's protocol. RNA quantitation
was performed using TaqMan real-time RT-PCR, relative mRNA abundance was
calculated using the comparative delta-Ct method with GAPDH mRNA as standard. Procedure
and primer sequence was previously described [19].
Immunohistochemical analysis.
Frozen brain coronal sections (10 μm)
were fixed with 4% PFA dissolved in PBS (pH 7.5) for 10 min, permeabilized with
0.5% Triton X-100 for 5 min. The sections were incubated with primary
anti-BMAL1 antibodies raised in guinea pig followed by incubation with donkey
anti-guinea pig secondary antibody labeled with DyLight488 (Jackson
ImmunoResearch laboratories), incubated for 1 min with
4'-6-Diamidino-2-phenylindole (DAPI, 300nM, Invitrogen), mounted under cover
slips using Fluoromount G media (SouthernBiotech). The slides were kept in the
dark at +4oC until use. Microphotographs were taken with the aid of
Leica DMR upright microscope equipped with Princeton Instruments MicroMax 5
MHz-cooled CCD camera and ImagePro software.
ROS
analysis.
ROS levels were determined in tissue extracts using
ROS sensitive fluorescent dye as described elsewhere [4]. Briefly, brain was
immediately frozen on dry ice and stored at -70o C until analysis.
After mixing with 10 volumes of homogenization buffer and normalizing by
protein content, brain extracts were mixed with dichlorodihydrofluorescein
(DCF) in homogenization buffer and incubated in the dark at 37 o C
for 30 min. Fluorescence at 495/535nm was measured using Victor2 Wallac
microplate reader (Perkin Elmer). At least 3 animals were used for analysis for
every time point and genotype.
Statistical
analysis.
Six male mice of each
genotype were used for all experiments. Data are shown as mean + standard
deviation. SigmaStat software package was used for analysis. Effects of
genotype (circadian mutants versus wild type) and novel/familiar environment
(day1 versus day2) on behavioral variables collected in open field experiments
were tested for significance with Two Way Repeated Measures ANOVA. Bonferoni
t-test was used for all pairwise multiple comparison procedures. Unpaired
Student's t-test was used for comparison of total activities between day1 and
day2 for the same genotype or the same day for different genotypes. Unpaired
Student's t-test was used for comparison of between genotype variations in
relative gene expression and ROS level at different time points. P<0.05 was
considered as statistically significant.
We
thank Yelena Kondratova for the editorial help. This work was supported by
start-up fund and AHA grant 0835155N to R.V.K. and NIH grants CA102522 and
GM075226 to M.P.A.
The authors of this manuscript have no conflict of
interests to declare.