To identify differentially regulated proteins upon chemotherapeutic treatment, hTERT-BJ1 fibroblasts were exposed for 48 h to either vehicle or sub-lethal concentrations of azathioprine (100 μM) or taxol (100 nM) (Figure S1), and cell lysates were subject to labelfree quantitative proteomics. Following protein digestion with trypsin, peptide fractions were processed on an LTQ-Orbitrap XL mass spectrometer. The experimental workflow used for the present study is depicted in Figure 2. Those peptides identified were further analyzed to find proteomic changes between chemotherapy-treated and vehicle-treated fibroblasts. To define differential regulation, those identified proteins that showed a fold change difference of 1.15 or higher, and p values of < 0.05 (ANOVA) compared to vehicle were considered. In the azathioprine-treated fibroblasts, 1640 proteins were identified as differentially expressed, from which 779 were upregulated and 861, down-regulated. In the taxol treatments, 2967 proteins were found as differentially expressed compared to vehicle, from which 1624 were up-regulated and 1343, down-regulated (Figure 3A).
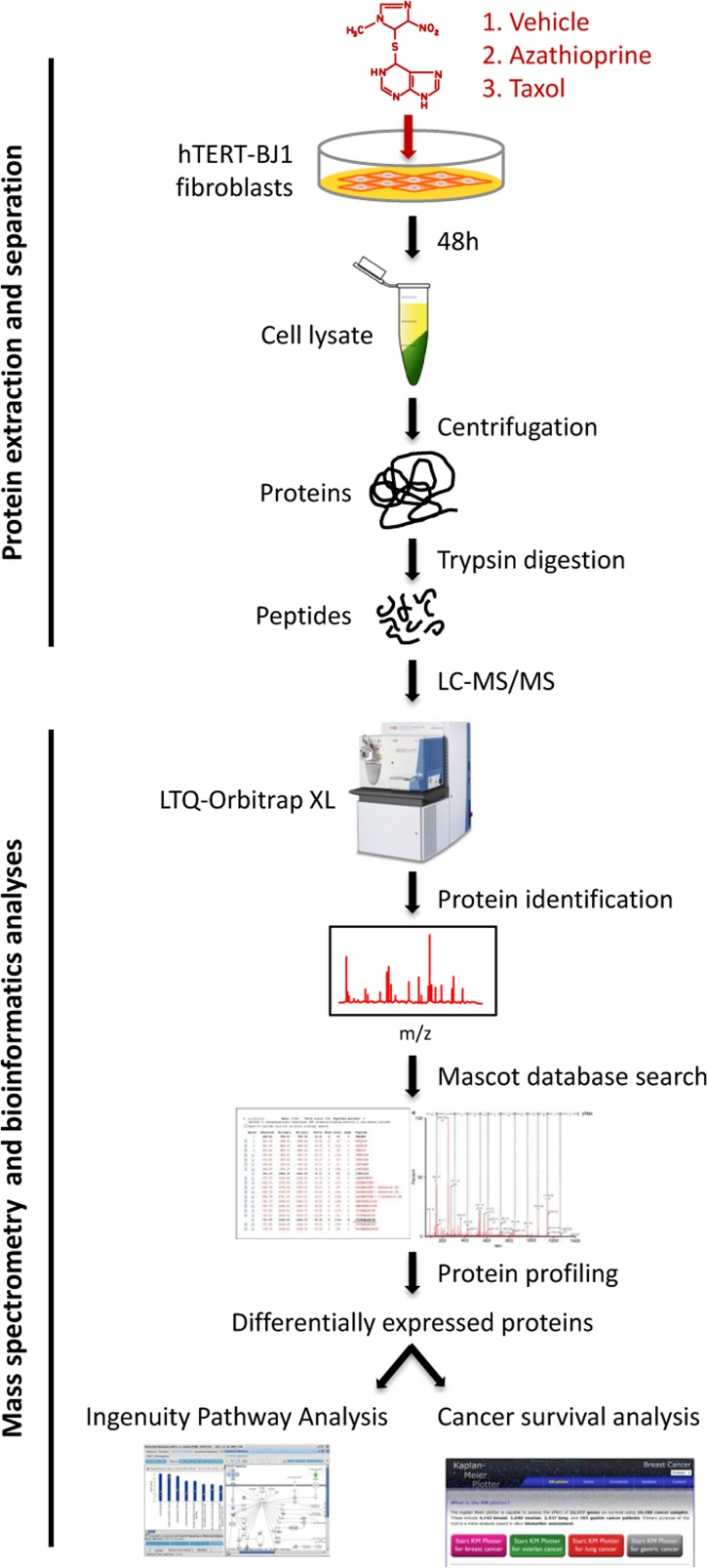
Figure 2. Workflow for the comparative proteome analysis of hTERT-BJ1 fibroblasts treated with azathioprine, taxol or vehicle. Protein lysates were obtained from hTERT-BJ1 fibroblasts after 48 h treatment with azathioprine, taxol or vehicle. Peptides obtained after trypsin digestion were analysed via LC-MS/MS on an LTQ-Orbitrap XL mass spectrometer. Label-free quantitative proteomics was used to detect changes in protein abundances across vehicle-treated and drug-treated fibroblast extracts. The proteomics data sets were further analysed using Ingenuity Pathway Analysis and a cancer survival analysis tool (kmplot.com).
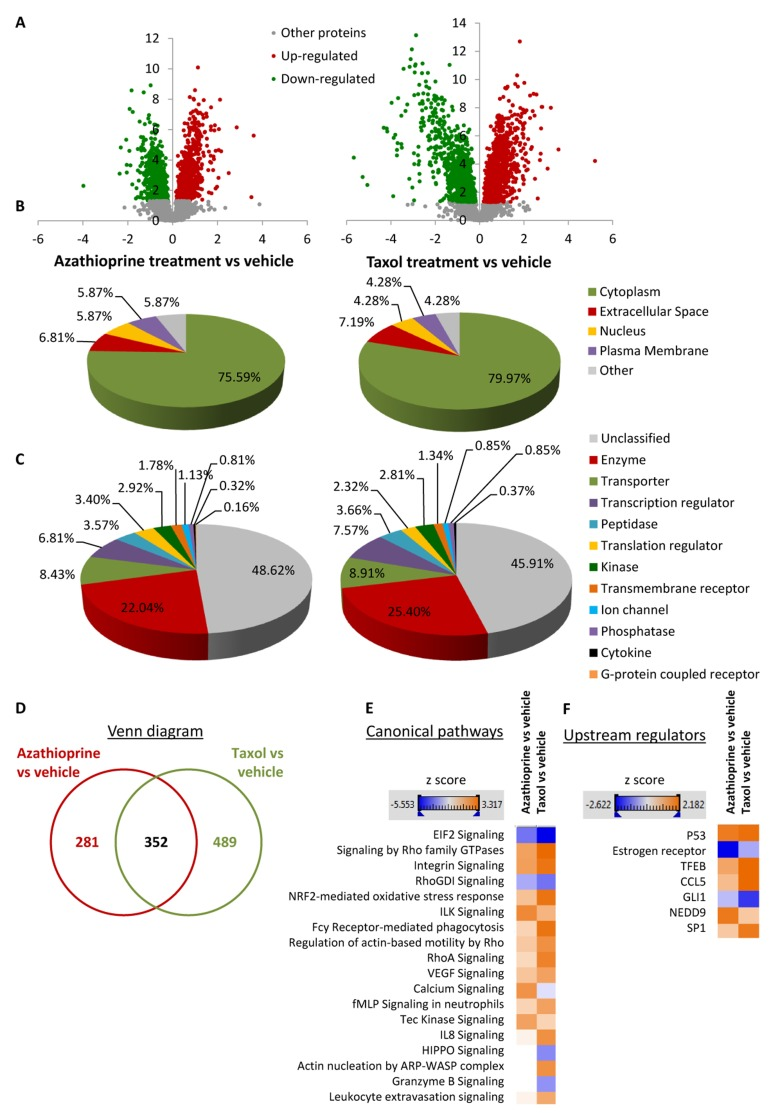
Figure 3. Overview of proteins and pathways identified as differentially regulated in the lysates of azathioprinetreated and taxol-treated fibroblasts relative to vehicle by Ingenuity Pathway Analysis. (A) Volcano plot representation of protein abundance changes in hTERT-BJ1 fibroblasts upon treatment with azathioprine and taxol compared to vehicle treatment. A total of 1640 differentially regulated proteins with fold changes ≥ 1.15 and p values < 0.05 were identified in azathioprine-treated fibroblasts, and 2967 differentially regulated proteins in taxol-treated fibroblasts. X axis represents log2(fold change). Y axis represents −log(p value). Non-significantly regulated proteins are shown in grey, in green, significantly down-regulated proteins and in red, significantly up-regulated proteins. (B) Subcellular localization of differentially regulated proteins identified in azathioprine and taxol treatments compared to vehicle treatment. (C) Classification of differentially regulated proteins identified in azathioprine and taxol treatments by type. Cellular enzymes account for 22.04% and 25.40% of total differentially regulated proteins identified in azathioprine treatment and taxol treatment, respectively. (D) Overlap of differentially regulated proteins identified in azathioprine and taxol treatments compared to vehicle treatment. Of all proteins identified by quantitative proteomics, 352 were proteins the expression of which was found altered in both treatments compared to vehicle. (E) Canonical pathways and (F) upstream regulators identified or predicted as altered in both treatment conditions compared to vehicle. A positive z score is indicated in orange and points towards an activation of the pathway, and a negative z score, in blue, indicates an inhibition of the pathway.
To obtain additional functional insights into pathways that are differentially regulated in stromal fibroblasts upon treatment, bioinformatics analyses of our proteomics datasets were conducted. All differentially expressed proteins were analysed using Ingenuity Pathway Analysis (IPA) to seek altered canonical pathways and toxicity functions. IPA was able to analyse 633 proteins out of 1640 in the azathioprine-treated fibroblasts, and 841 out of 2967 proteins in the taxol-treated fibroblasts. We further examined the subcellular distribution and the nature of these differentially regulated proteins in azathioprine and taxol-treated fibroblasts. Over 80% of all proteins analyzed were intracellular, in particular cytoplasmic proteins (Figure 3B). Likewise, the largest portion of classified proteins, accounting for one fourth of all analysed proteins, were enzymes, over 8% of all proteins were transporter proteins, and over 7% were transcription regulators (Figure 3C). Finally, a comparison analysis revealed that 352 proteins were differentially regulated in both treatment conditions compared to vehicle (Figure 3D).
Cellular pathways affected by chemotherapy in stromal fibroblasts
1 Metabolism
One of the major contributors to the aging process is mitochondrial dysfunction, which involves a decrease in the oxidative phosphorylation efficacy and an increase in the electron leakage resulting in reduced ATP generation [17]. Similarly, one of the hallmarks of the catabolic tumour stroma is the induction of metabolic stress that favours glycolysis to the detriment of mitochondrial metabolism. We have showed in a previous study that several chemotherapeutic agents, including azathioprine and taxol were able to stimulate stromal fibroblasts to consume more glucose and produce more lactate, which was released via enhanced MCT4 expression, hence increasing extracellular acidification (Figure 1). The cellular ATP content was also minor upon treatment suggesting a decrease in mitochondrial respiration [11].
To validate that chemotherapy-induced glycolytic phenotype and to identify other cellular metabolic pathways potentially altered by chemotherapy, we searched the proteomic data for changes in the expression of metabolic enzymes. Of all differentially regulated proteins, 22.04% and 25.40% were actually enzymes in azathioprine-treated and taxol-treated fibroblasts, respectively (Figure 3C).
The expression of most glycolytic enzymes was significantly altered (Table 1). Interestingly, most of the glycolytic enzymes that were found to be up-regulated are enzymes that perform an irreversible reaction in the glycolytic pathway and all down-regulated enzymes are reversible enzymes, able to perform the opposite reaction, hence also involved in gluconeogenesis. Curiously, LDHA, the enzyme involved in transforming pyruvate into lactate was found to be down-regulated in both treatments suggesting that pyruvate was further processed into the TCA cycle instead of being transformed into lactate.
Table 1. Changes in the expression of enzymes involved in glucose metabolism after treatment with azathioprine and taxol for 48 h as measured by quantitative proteomics
| GLYCOLYSIS | | Azathioprine | Taxol |
| Solute carrier family 2 (facilitated glucose transporter), member 1 (GLUT1) | SLC2A1 | ↑ 1.18 | |
| Hexokinase | HK1 | | ↑ 1.43 |
| HK2 | ↑ 2.46 | |
| Glucose-6-phosphate isomerase | GPI | | ↓ 1.27 |
| 6-phosphofructokinase type C | PFKP | | ↑ 1.56 |
| Fructose-bisphosphate aldolase A | ALDOA | ↑ 2.10 | ↓ 11.58 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | ↑ 1.90 | ↑ 1.89 |
| Triosephosphate isomerase 1 | TPI1 | | ↑ 1.33 |
| Phosphoglycerate kinase 1 | PGK1 | | ↓ 1.58 |
| Phosphoglycerate mutase | PGAM1 | | ↓ 1.38 |
| PGAM4 | ↓ 1.45 | |
| Enolase 1 | ENO1 | ↓ 1.37 | |
| Pyruvate kinase | PKM | | ↑ 1.65 |
| POST-GLYCOLYSIS PROCESSES | | Azathioprine | Taxol |
| L-lactate dehydrogenase A chain | LDHA | ↓ 1.47 | ↓ 1.46 |
| Monocarboxylate transporter 4 (MCT4) | SLC16A3 | | ↑ 2.21 |
| Pyruvate dehydrogenase | PDHB | ↓ 1.20 | ↓ 1.40 |
| GLUCONEOGENESIS | | Azathioprine | Taxol |
| Glucose-6-phosphate isomerase | GPI | | ↓ 1.27 |
| Aldolase A, fructose-bisphosphate | ALDOA | ↑ 2.10 | ↓ 11.57 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | ↑ 1.90 | ↑ 1.89 |
| Phosphoglycerate kinase 1 | PGK1 | | ↓ 1.58 |
| Phosphoglycerate mutase | PGAM1 | | ↓ 1.38 |
| PGAM4 | ↓ 1.45 | |
| Enolase 1 | ENO1 | ↓ 1.37 | |
| Malate dehydrogenase | MDH1 | | ↓ 1.80 |
| MDH2 | | ↓ 1.23 |
| Malic enzyme 2, NAD(+)-dependent, mitochondrial | ME2 | | ↓ 1.62 |
| PENTOSE PHOSPHATE PATHWAY | | Azathioprine | Taxol |
| Glucose-6-phosphate dehydrogenase | G6PD | ↓ 2.03 | ↑ 2.69 |
| H6PD | ↑ 1.46 | |
| 6-phosphogluconolactonase | PGLS | | ↑ 1.54 |
| Phosphogluconate dehydrogenase | PGD | | ↓ 1.57 |
| Transketolase | TKT | ↓ 1.88 | |
| Transaldolase | TALDO | ↑ 2.13 | |
| HEXOSAMINE BIOSYNTHESIS PATHWAY | | Azathioprine | Taxol |
| Glutamine-fructose-6-phosphate transaminase 1 | GFPT1 | | ↑ 1.36 |
| Glucosamine-6-phosphate deaminase 1 | GNPDA1 | | ↑ 2.46 |
| Chemotherapy increased the expression of several enzymes involved in glycolysis, pentose phosphate pathway, and hexosamine biosynthesis. Enzymes that show amplified expression after treatment are shown in red, and enzymes that are decreased are shown in green. Number indicates the fold increase or fold decrease in protein expression in chemotherapy-treated versus vehicle-treated hTERT-BJ1 fibroblasts. |
Nevertheless, LDH is an enzyme that exhibits feedback inhibition, by which high lactate concentrations can suppress it. In fact, MCT4, the monocarboxylate transporter responsible for the secretion of lactate, turned out to be up-regulated in taxol-treated hTERT-BJ1 cells, and regarding the further processing of pyruvate into acetyl-CoA and the citric acid (TCA) cycle, the expression of practically all enzymes were found to be down-regulated in taxol-treated fibroblasts compared to vehicle indicating a dramatic down-regulation of mitochondrial metabolism. A similar down-regulation trend was observed in numerous proteins involved in all oxidative phosphorylation complexes (Table 2) and mitochondrial function proteins (Table S1). Finally, several enzymes involved in mitochondrial fatty acid β-oxidation were also identified as down-regulated, and a few enzymes involved in fatty acid biosynthesis, up-regulated (Table S2). Other metabolic changes included enzymes of the oxidative pentose phosphate pathway, responsible for the generation of antioxidant power (NADPH), the hexosamine synthesis pathway, accountable for the production of amino sugars used for the synthesis of glycoproteins, glycolipids and proteoglycans, and a few enzymes involved in the generation of ketone bodies (Table 1 and 5). Figure 4A summarises all changes observed in the expression of metabolic enzymes in taxol-treated fibroblasts and their contribution to different cellular metabolic pathways.
Table 2. Changes in the expression of enzymes involved in mitochondrial glucose metabolism after treatment with azathioprine and taxol for 48 h as measured by quantitative proteomics
| TCA CYCLE | | Azathioprine | Taxol |
| Citrate synthase, mitochondrial | CS | | ↓ 1.52 |
| Aconitate hydratase | ACO2 | ↑ 1.41 | ↓ 1.23 |
| Dihydrolipoyl dehydrogenase | DLD | | ↓ 1.48 |
| Alpha-ketoglutarate dehydrogenase complex dihydrolipoyl succinyltransferase | DLST | ↓ 1.23 | ↓ 1.70 |
| 2-oxoglutarate dehydrogenase | OGDH | ↓ 1.37 | |
| Beta-succinyl CoA synthetase | SUCLA2 | | ↓ 1.25 |
| Succinate dehydrogenase complex | SDHA | | ↓ 2.43 |
| SDHB | ↓ 1.51 | |
| Fumarate hydratase | FH | | ↓1.27 |
| Malate dehydrogenase | MDH1 | | ↓ 1.80 |
| MDH2 | | ↓ 1.22 |
| OXIDATIVE PHOSPHORYLATION | | Azathioprine | Taxol |
| NADH dehydrogenase (complex I) | NDUFV1 | | ↑ 1.54 |
| NDUFV2 | | ↓ 1.45 |
| NDUFS1 | | ↓ 1.73 |
| NDUFS7 | | ↓ 1.31 |
| NDUFB10 | | ↓ 1.29 |
| Succinate dehydrogenase complex (complex II) | SDHA | | ↓ 2.43 |
| SDHB | ↓ 1.51 | |
| Coenzyme Q – cytochrome c reductase (complex III) | CYCS | | ↓ 1.61 |
| UQCRC1 | | ↓ 1.42 |
| UQCRB | | ↑ 1.62 |
| Cytochrome c oxidase (complex IV) | COX17 | | ↓ 2.00 |
| COX6C | | ↓ 1.62 |
| COX6A1 | ↑ 1.47 | |
| COX5A | | ↓ 1.39 |
| COX5B | ↑ 1.37 | |
| ATP synthase (complex V) | ATP5A1 | ↓ 1.51 | ↓ 1.16 |
| ATP5F1 | ↓ 1.70 | ↓ 1.51 |
| ATP5H | ↑ 1.56 | |
| ATP5J | ↑ 1.36 | |
| ATP5B | | ↑ 2.36 |
| Taxol treatment remarkably decreased the expression of enzymes of the TCA cycle and oxidative phosphorylation. Enzymes that show increased expression after treatment are shown in red, and enzymes that are decreased are shown in green. Number indicates the fold increase or fold decrease in protein expression in chemotherapy-treated versus vehicle-treated hTERT-BJ1 fibroblasts. |
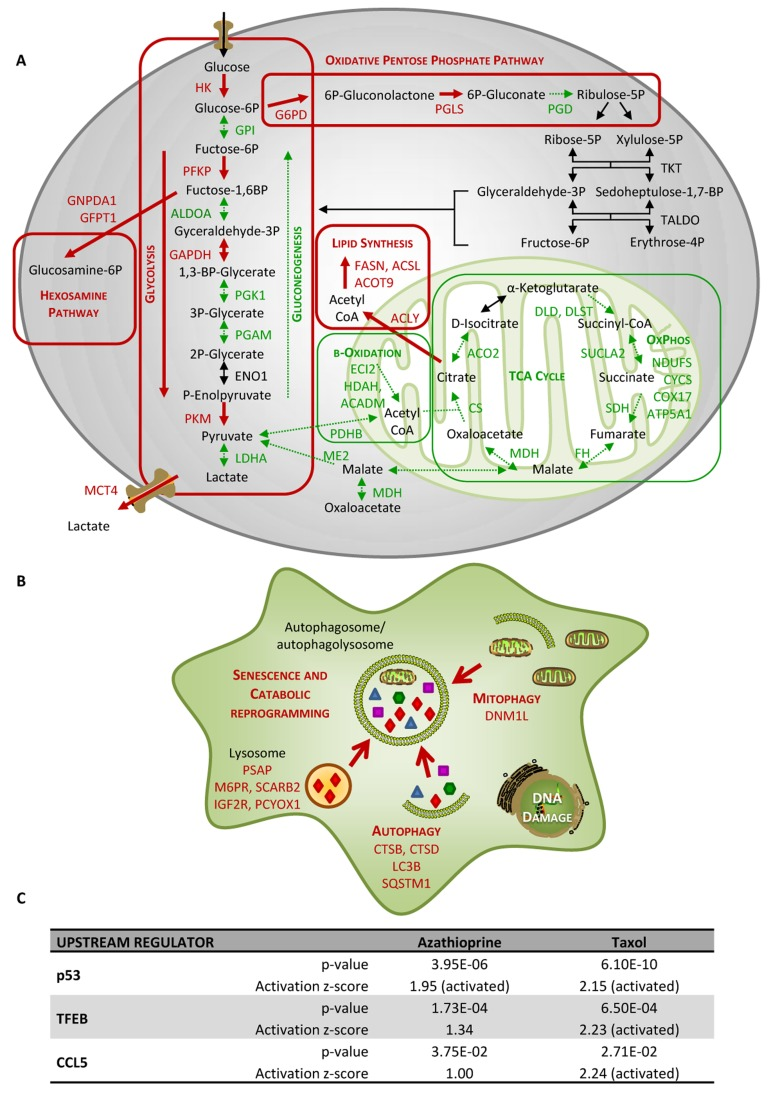
Figure 4. Taxol treatment alters several cellular metabolic pathways and induces autophagy and senescence in hTERTBJ1 fibroblasts. (A) Summary of changes observed in the expression of numerous metabolic enzymes after treatment with taxol for 48 h, as measured by quantitative proteomics. Taxol treatment increased the expression of enzymes involved in glycolysis, pentose phosphate, and hexosamine biosynthesis and lipid synthesis pathways in detriment of those involved in gluconeogenesis and mitochondrial metabolism (TCA cycle, oxidative phosphorylation, and mitochondrial β–oxidation). Enzymes that show amplified expression after taxol treatment are shown in red, and enzymes that are decreased are shown in green. Cellular metabolic pathways that may be up-regulated are boxed in red, and those that may be down-regulated are boxed in green. (B) Summary of changes observed in the expression of numerous autophagy and senescence-related proteins after treatment with taxol for 48 h, as measured by quantitative proteomics. Taxol amplified the expression of proteins involved in senescence, autophagy, mitophagy and vesicle formation and trafficking. Proteins that show increased expression after treatment are shown in red. (C) Ingenuity Pathway Analysis of azathioprine and taxol-treated hTERT-BJ1 fibroblasts predicted p53, TFEB and CCL5 to be activated in these cells.
IPA revealed glycolysis, gluconeogenesis and pentose phosphate pathway as altered canonical pathways in both azathioprine and taxol treatments. Similarly, IPA revealed mitochondrial dysfunction, TCA cycle, and PPARα/RXRα activation, responsible for ketone body production and fatty acid metabolism, as two of the top canonical pathways affected by both treatments, and oxidative phosphorylation and fatty acid β-oxidation, as altered pathways also in taxol-treated cells (Figure 5 and Table S5). The toxicity impact of both drugs extensively involved mitochondrial dysfunction and damage, and also fatty acid metabolism and PPARα/RXRα activation (Figure 6 and Table S6).
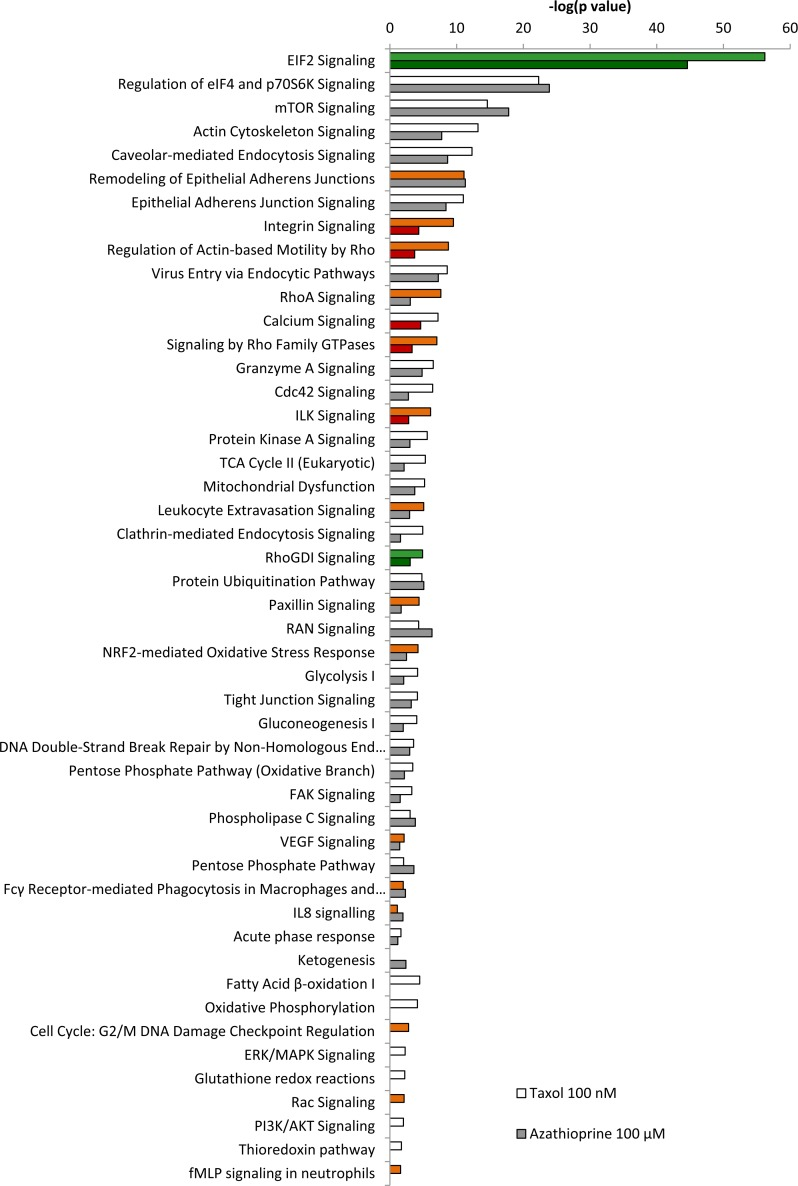
Figure 5. Pathway analysis of differentially expressed proteins in hTERT-BJ1 fibroblasts treated with azathioprine or taxol compared to vehicle-treated cells. Ingenuity Pathway Analysis showed canonical pathways significantly altered by the proteins differentially expressed in hTERT-BJ1 fibroblasts treated with azathioprine or taxol (P < 0.05). The p value for each pathway is indicated by the bar and is expressed as −1 times the log of the p value. Green coloured bars show a predicted inhibition of the pathway (z score <-1.9) and red coloured bars indicate a predicted activation of the pathway (z score > 1.9).
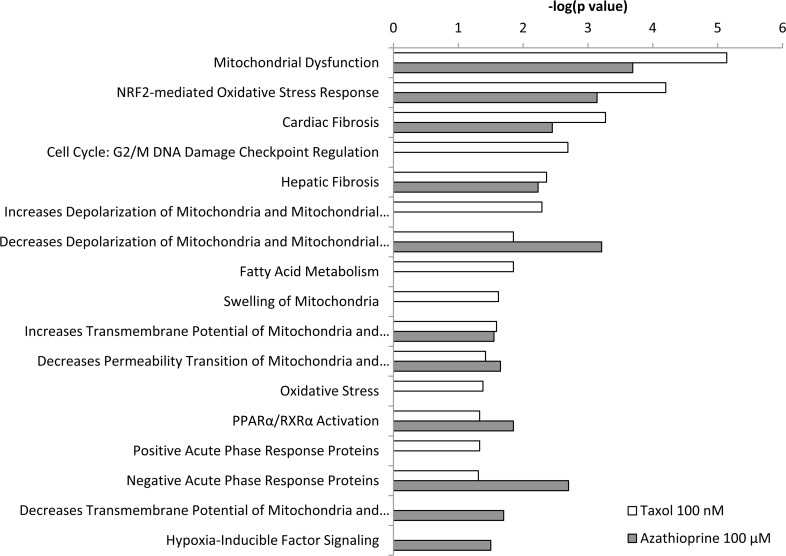
Figure 6. Toxicity effects of differentially expressed proteins in hTERT-BJ1 fibroblasts treated with azathioprine or taxol compared to vehicle-treated cells. Ingenuity Pathway Analysis showed toxicity functions significantly enriched by the proteins differentially expressed in hTERT-BJ1 fibroblasts treated with azathioprine or taxol (P < 0.05). The p value for each pathway is indicated by the bar and is expressed as −1 times the log of the p value.
To recapitulate, the previously identified chemotherapy-induced metabolic stress in stromal fibroblasts is clearly detected by quantitative proteomics and it represents not only an increase in glycolysis and a reduction in mitochondrial function, as observed in our previous study, but also affects other metabolic pathways, including hexosamine synthesis and pentose phosphate pathways, fatty acid metabolism and ketogenesis.
2 Antioxidant response and stress-related pathways
Another feature of the catabolic tumour stroma is the induction of oxidative stress, which can be induced by chemotherapy in cancer cells and also healthy tissues (Figure 1) [18, 19]. Particularly, we previously showed increased ROS production and antioxidant response in hTERT-BJ1 fibroblasts after treatment with taxol [11]. We have seen in this study that several enzymes of the oxidative branch of the pentose phosphate pathway, which is responsible for the generation of antioxidant power (NADPH), were up-regulated in chemotherapy-treated stromal fibroblasts. Thus, we sought for other antioxidant response proteins in the proteomics datasets. The expression of numerous proteins involved in Nrf2-mediated antioxidant response and other antioxidant proteins was significantly altered, in most cases up-regulated (Table 3), suggesting an activation of the pathway in both azathioprine and taxol-treated hTERT-BJ1 fibroblasts, although it was found to be notably higher in taxol-treated cells.
Table 3. Changes in the expression of Nrf2-target proteins and other proteins involved in the antioxidant response after treatment with azathioprine and taxol for 48 h as measured by quantitative proteomics and indicated by IPA analysis
| NRF2-MEDIATED ANTIOXIDANT RESPONSE | | Azathioprine | Taxol |
| ATP-binding cassette, sub-family C (CFTR/MRP), member 1 | ABCC1 | ↓ 2.34 | |
| Actin, alpha 2, smooth muscle, aorta | ACTA2 | ↑ 1.34 | ↑ 1.78 |
| Actin, beta | ACTB | | ↑ 1.30 |
| Actin, alpha, cardiac muscle 1 | ACTC1 | ↑ 1.75 | ↑ 1.20 |
| Carbonyl reductase 1 | CBR1 | | ↑ 1.29 |
| Chaperonin containing TCP1, subunit 7 (eta) | CCT7 | ↑ 2.89 | |
| DnaJ (Hsp40) homolog | DNAJA1 | ↑ 1.61 | ↓ 9.61 |
| DNAJB11 | ↓ 1.34 | |
| DNAJC8 | ↓ 1.79 | ↓ 1.58 |
| DNAJC13 | | ↓ 4.30 |
| Epoxide hydrolase 1, microsomal (xenobiotic) | EPHX1 | | ↑ 2.06 |
| Ferritin, light polypeptide | FTL | ↑ 1.61 | ↑ 6.97 |
| Glutathione S-transferase | GSTK1 | | ↑ 1.34 |
| GSTO1 | | ↑ 1.57 |
| GSTP1 | | ↓ 1.53 |
| Heme oxygenase (decycling) 1 | HMOX1 | ↑ 4.48 | ↑ 2.83 |
| Mitogen-activated protein kinase 3 | MAPK3 | ↑ 1.54 | |
| Peptidylprolyl isomerase B (cyclophilin B) | PPIB | ↑ 1.60 | ↑ 1.36 |
| Peroxiredoxin 1 | PRDX1 | | ↑ 1.68 |
| Protein kinase C, alpha | PRKCA | ↑ 1.96 | |
| Related RAS viral (r-ras) oncogene homolog | RRAS | | ↑ 2.44 |
| Superoxide dismutase 1, soluble | SOD1 | | ↑ 1.48 |
| Superoxide dismutase 2, mitochondrial SOD2 | | ↑ 1.69 | ↓ 1.87 |
| Sequestosome 1 | SQSTM1 | | ↑ 3.50 |
| Thioredoxin TXN | | | ↑ 2.75 |
| Thioredoxin reductase 1 | TXNRD1 | | ↑ 2.66 |
| Valosin-containing protein VCP | | ↓ 1.29 | |
| ANTIOXIDANTS (OTHER) | | Azathioprine | Taxol |
| Aminopeptidase N | ANPEP | ↑ 1.78 | ↑ 1.56 |
| Glutaredoxin-1 | GLRX | | ↑ 1.25 |
| Glutathione peroxidase | GPX8 | | ↓ 1.85 |
| Peroxidasin | PXDN | | ↑ 3.57 |
| PRDX4 | ↑ 1.50 | |
| Peroxiredoxin | PRDX5 | | ↑ 1.33 |
| PRDX6 | | ↓ 1.23 |
| Chemotherapy mostly increased the expression of proteins involved in antioxidant response, mainly in taxol-treated fibroblasts. Proteins that show amplified expression after treatment are shown in red, and proteins that are decreased are shown in green. Number indicates the fold increase or fold decrease in protein expression in chemotherapy-treated versus vehicle-treated hTERT-BJ1 fibroblasts. |
A higher presence of antioxidant response proteins was further confirmed using IPA, which revealed Nrf2-mediated antioxidant response to be one of the top canonical pathways affected by azathioprine and taxol treatments, although z score values demonstrated a significant activation of this pathway exclusively in taxol-treated fibroblasts. Similarly, taxol-treated cells showed altered glutathione and thioredoxin antioxidant pathways (Figure 3E and 5, and Table S5). The toxicity of these chemotherapeutic drugs also involved oxidative stress. HIF signalling, which is activated in response to stress and a central player in the regulation of cellular metabolism, was also identified as a toxic effect of azathioprine treatment (Figure 6 and Table S6), and had been previously identified as activated in hTERT-BJ1 fibroblasts treated with azathioprine and taxol [11]. Thus, proteomics analysis clearly detects an activation of the antioxidant response after treatment with chemotherapy.
3 Myofibroblastic differentiation
Cancer-associated fibroblasts are commonly identified by their expression of alpha smooth muscle actin (αSMA) [20] (Figure 1). Indeed, we previously showed up-regulation of αSMA by immunoblotting in taxol-treated hTERT-BJ1 [11]. Quantitative proteomics profiling of azathioprine and taxol-treated hTERT-BJ1 fibroblasts also revealed a significantly higher presence of αSMA and many other myofibroblastic markers, such as fibroblast activation protein (FAP) or vimentin, as well as muscle-related proteins compared to the vehicle-treated control (Table 4).
Table 4. Changes in the expression of proteins involved in myofibroblastic transformation and muscle-related proteins after treatment with azathioprine and taxol for 48 h as measured by quantitative proteomics
| CANCER-ASSOCIATED FIBROBLAST AND MUSCLE-RELATED PROTEINS | | Azathioprine | Taxol |
|---|
| Actin, alpha, cardiac muscle | ACTC1 | ↑ 1.75 | ↑ 1.20 |
| Actin, alpha 2, aortic smooth muscle (αSMA) | ACTA2 | ↑ 1.34 | ↑ 1.78 |
| Caldesmon | CALD1 | | ↑ 2.49 |
| Calponin | CNN1 | | ↑ 2.76 |
| CNN2 | ↑ 1.81 | ↑ 2.76 |
| CNN3 | ↓ 1.28 | ↑ 2.46 |
| Desmin | DES | ↑ 1.21 | ↓ 1.79 |
| Dysferlin | DYSF | | ↑ 1.24 |
| Fibroblast activation protein | FAP | ↑ 1.74 | ↑ 1.56 |
| Fibronectin 1 | FN1 | ↑ 2.02 | ↑ 2.03 |
| Filamin | FLNA | ↓ 1.92 | ↑ Infinity |
| FLNB | ↓ 1.23 | |
| FLNC | | ↑ 1.56 |
| Moesin | MSN | ↑ 1.37 | ↑ 1.60 |
| Myoferlin MYOF | | ↑ 4.15 | ↑ 1.95 |
| Myosin | MYH4 | | ↑ 1.62 |
| MYH9 | ↑ 1.64 | |
| MYH10 | ↑ 1.37 | |
| MYH11 | ↑ 1.47 | ↑ 1.90 |
| MYH14 | ↓ 3.06 | |
| MYO1B | | ↓ 1.72 |
| MYO1C | ↑ 2.21 | ↑ 1.59 |
| MYO10 | ↑ 1.37 | |
| MYO18B | ↓ 1.50 | ↑ 2.62 |
| Myosin, light polypeptide kinase | MYLK | | ↑ 1.88 |
| Myosin phosphatase Rho interacting protein | MPRIP | | ↑ 1.45 |
| Myosin regulatory light polipeptides | MYL6 | ↑ 1.33 | ↑ 1.74 |
| MYL9 | | ↑ 1.77 |
| MYL12A | | ↑ 2.21 |
| Palladin | PALLD | | ↑ 3.88 |
| Platelet-derived growth factor receptor, beta | PDGFRB | | ↑ 1.59 |
| Prolyl 4-hydroxylase | P4HA1 | | ↑ 3.14 |
| P4HA2 | ↓ 1.32 | ↓ 2.50 |
| P4HB | ↓ 1.76 | |
| Talin | TLN1 | ↑ 1.46 | ↑ 2.95 |
| TLN2 | ↓ 1.80 | |
| Transgelin 2 | TAGLN2 | ↑ 1.93 |
| Tropomyosin | TPM1 | ↓ 2.63 | ↓ 4.23 |
| TPM2 | ↑ 1.24 | |
| TPM3 | ↑ 1.43 | |
| TPM4 | | ↑ 2.12 |
| Vimentin | VIM | ↑ 1.72 | ↑ 1.76 |
| Chemotherapy enormously increased the expression of proteins involved in CAF transformation. Proteins that show amplified expression after treatment are shown in red, and proteins that are decreased are shown in green. Number indicates the fold increase or fold decrease in protein expression in chemotherapy-treated versus vehicle-treated hTERT-BJ1 fibroblasts. |
Myofibroblasts are mostly responsible for the presence of fibrosis [21], and the toxicity functions of these chemotherapeutic drugs involved tissue fibrosis, as analysed by IPA (Figure 6 and Table S6). Therefore, chemotherapy can independently induce the differentiation of hTERT-BJ1 fibroblasts into cancer-associated fibroblasts, which can be detected by a numerous increase of myofibroblastic markers in quantitative proteomics analysis.
4 Autophagy and senescence
Autophagy and senescence represent a common response to stresses such as exposure to DNA-damaging exogenous cytotoxic agents, including chemotherapy or radiation [22, 23]. Indeed our previous study indicated that azathioprine and taxol induce autophagic vesicle formation and increase β-galactosidase activity in stromal fibroblasts [11]. Thus, to further examine changes in autophagy and senescence upon chemotherapeutic exposure, the differential expression of several autophagy, mitophagy and lysosomal markers was analysed using the proteomics datasets. Numerous autophagy and senescence markers such as sequestrosome 1, also known as p62, cathepsin B or the lysosomal enzyme β-galactosidase were found to be up-regulated in either azathioprine or taxol treatments (Table 5). In addition, DNM1L, a protein involved in mitochondrial fission, was up-regulated in taxol-treated hTERT-BJ1 fibroblasts, and OPA1, a protein involved in mitochondrial fusion, was down-regulated in azathioprine-treated cells. Likewise, VAT1, a vesicle membrane protein that inhibits mitochondrial fusion, was up-regulated in both treatments, suggesting that mitophagy might be also activated in response to chemotherapy (Table 5 and Table S3).
Table 5. Changes in the expression of autophagy, mitophagy and senescence markers and ketogenesis enzymes after treatment with azathioprine and taxol for 48 h as measured by quantitative proteomics
| AUTOPHAGY MARKERS | | Azathioprine | Taxol |
| Cathepsin | CATB | ↑ 1.56 | ↑ 1.55 |
| CATD | | ↑ 1.62 |
| Lysosomal-associated membrane protein 1 | LAMP1 | ↑ 1.55 | |
| Microtubule-associated protein 1 light chain 3 beta | MAP1LC3B | | ↑ 1.58 |
| Sequestrosome 1 (p62) | SQSTM1 | | ↑ 3.50 |
| MITOPHAGY MARKERS | | Azathioprine | Taxol |
| Dynamin-1-like protein (fission) | DNM1L | | ↑ 1.71 |
| Dynamin-like 120 kDa protein, mitochondrial (fusion) | OPA1 | ↓ 1.44 | |
| LYSOSOMAL PROTEINS (OTHER) | | Azathioprine | Taxol |
| Cation-dependent mannose-6-phosphate receptor | M6PR | | ↑ 2.38 |
| Cation-independent mannose-6-phosphate receptor | IGF2R | ↑ 3.58 | ↑ 1.88 |
| Galactosidase, beta 1 | GLB1 | ↑ 1.53 | |
| Late endosomal/lysosomal adaptor, MAPK and mTOR activator 1 | LAMTOR1 | ↑ 1.85 | |
| Lysosome membrane protein 2 | SCARB2 | ↑ 1.63 | ↑ 1.49 |
| N-acetylglucosamine-6-sulfatase | GNS | | ↑ 1.43 |
| Prenylcysteine oxidase 1 | PCYOX1 | | ↑ 1.55 |
| Prosaposin | PSAP | ↑ 2.21 | ↑ 2.13 |
| KETOGENESIS | | Azathioprine | Taxol |
| Hydroxyacyl-CoA dehydrogenase | HADH | | ↓ 1.43 |
| HADHA | ↓ 1.57 | |
| HADHB | ↑ 1.36 | |
| 3-Hydroxymethyl-3-methylglutaryl-CoA lyase | HMGCL | ↑ 1.67 | |
| Chemotherapy incremented the expression of proteins involved in autophagy and senescence. Proteins that show increased expression after treatment are shown in red, and proteins that are decreased are shown in green. Number indicates the fold increase or fold decrease in protein expression in chemotherapy-treated versus vehicle-treated hTERT-BJ1 fibroblasts. |
During autophagy, protein and lipid degradation occur, the latter leading to the generation of ketone bodies. A long list of up-regulated proteins involved in vesicle formation and trafficking, in protein ubiquitination pathway and proteasomal degradation, and a few enzymes involved in ketogenesis were also identified (Table 5, S3 and S4). The boost in vesicle formation and trafficking proteins could explain the greater presence of enzymes involved in fatty acid synthesis and hexosamine biosynthesis (Figure 4A and Table 1 and S2). The observed effects of taxol treatment on autophagy, mitophagy and senescence are summarised in Figure 4B.
IPA analysis confirmed an alteration of ketogenesis in fibroblasts exposed to azathioprine (Figure 5 and Table S5). IPA also showed a robust inhibitory effect of azathioprine and taxol on EIF2 signalling, responsible for protein synthesis, as measured by z score, and also revealed an alteration in the regulation of eIF4 and p70S6K signaling and in the protein ubiquitination pathway. Finally, PPARα/RXRα activation, Rho, caveolar and clathrin-mediated signalling pathways, all involved in vesicle trafficking and motility, and the mTOR signalling, known for its role in autophagy, mitochondrial metabolism and lipid metabolism, as well as cytoskeleton dynamics, were some of the top canonical pathways altered by chemotherapy, (Figure 3E, 5, and Table S5). Interestingly, p53, known mediator of senescence, was predicted to be activated in both azathioprine-treated and taxol-treated hTERT-BJ1 cells according to IPA, and TFEB, a transcriptor factor that coordinates the expression of lysosomal hydrolases, membrane proteins and genes involved in autophagy, was also predicted to be activated by taxol treatment (Figure 3F and 4C). Our previous study also reported an increased expression of p53 in hTERT-BJ1 fibroblasts treated with azatioprine and taxol by immunoblotting [11].
Thus, quantitative proteomics analysis reveals a higher presence of markers of autophagy and senescence and proteins involved in protein degradation and vesicle trafficking in hTERT-BJ1 fibroblasts, upon treatment.
5 Inflammation
Senescent cells dramatically alter their secretome, enriching it with pro-inflammatory cytokines and matrix metalloproteinases. This senescence-associated secretory phenotype (SASP) can lead to chronic inflammation, which is a hallmark of aging [17]. Stromal fibroblasts secrete inflammatory cytokines when in contact with cancer cells [24]. Chemotherapy is also able to induce cytokine production in healthy tissues [12], and in particular taxol treatment induces IL6 secretion in stromal fibroblasts (Figure 1) [11]. STAT3, a known inducer of inflammation in response to stress [25, 26] was found significantly up-regulated in both azathioprine (1.69 fold increase) and taxol treatments (2.35 fold increase) relative to vehicle. STAT3 signalling was found to be activated in response to taxol also in our previous study [11].
Interestingly, several pathways involved in the inflammatory process such as IL8, acute phase response, leukocyte extravasation or Fcγ receptor-mediated phagocytosis signalling were amongst altered canonical pathways in both azathioprine-treated and taxol-treated hTERT-BJ1 cells relative to vehicle, according to IPA. N-formyl-Met-Leu-Phe (fMLP) signalling was also found to be altered in taxol-treated fibroblasts (Figure 3E and 5 and Table S5). Most of these pathways were clearly activated in taxol-treated fibroblasts as indicated by z score values, suggesting an induction of the inflammatory response in chemotherapy-treated stromal fibroblasts. Likewise, the chemokine (C-C motif) ligand 5 (CCL5 or RANTES), which plays a role in recruiting leukocytes into inflammatory sites, was identified as an upstream regulator in both treatments, particularly activated in taxol-treated fibroblasts (Figure 3F and 4C). Finally, acute phase response, which occurs soon after the onset of an inflammatory process, was identified as one of the toxic effects of both drugs (Figure 6 and Table S6). Therefore, pathway analysis of the proteomics results indicates an induction of the inflammatory response in stromal fibroblasts after exposure to chemotherapy.
Differentially up-regulated proteins in taxol-treated fibroblasts correlate with recurrence, metastasis and poor cancer survival
To further investigate the clinical implications of our proteomics datasets, we decided to test the impact of over-expressed proteins in taxol-treated hTERT-BJ1 fibroblasts in cancer prognosis. To do so, we used an on-line survival analysis tool that uses microarray gene expression data from multiple studies on breast, ovarian, lung and gastric cancer [16], which was suitable to our purpose since taxol is a chemotherapeutical drug currently used as therapy for most of these malignancies. Only those proteins with a fold change difference of 1.75 or higher and p values of < 0.05 (ANOVA) compared to vehicle were used for survival analyses. The expression of several proteins was found to correlate with survival in breast, ovarian, gastric and lung cancer patients. In particular, high expression of ubiquitin-like modifier activating enzyme 1 (UBA1), implicated in protein catabolism and degradation, showed a striking correlation with poor relapse-free survival, distant metastasis-free survival and overall survival in breast cancer patients previously treated with chemotherapy (425, 122 and 69 patients, respectively) (Figure 7A). The same correlation was not observed when patients who did not receive systemic treatment were considered (1000, 533 and 375 patients, respectively) (Figure S2A). Similarly, high expression of UBA1 correlated with poor overall survival in lung and gastric cancer patients previously treated with chemotherapeutic drugs (176 and 153 patients, respectively), and with progression-free survival in ovarian cancer patients particularly treated with paclitaxel (229 patients) (Figure 8A). Once more, the correlation was lost when patients who did not receive systemic treatment were considered in lung cancer (227 patients) or when patients who underwent only surgery were examined in gastric cancer (174 patients) (Figure S3A). No data from untreated ovarian cancer patients was available.
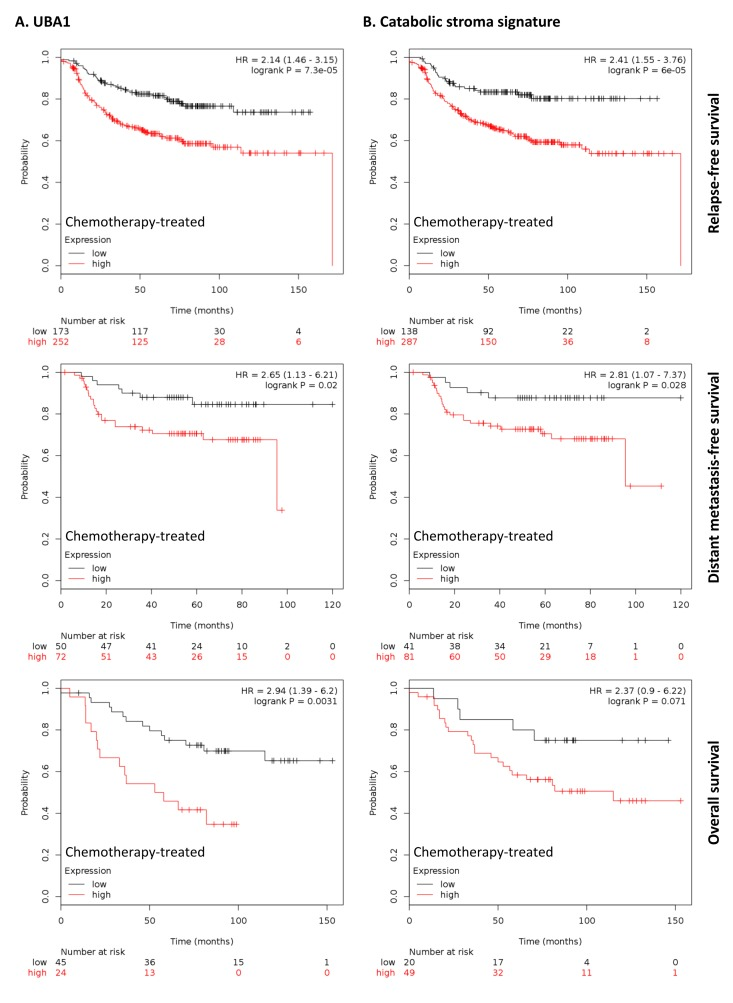
Figure 7. Clinical correlations of UBA1 expression and the UBA1, PSMC5, IGF2R, VAT1, HMOX1, CNN2, TLN1, GNPDA1, G6PD and FASN signature in chemotherapy-treated breast cancer patients. (A) Correlations of UBA1 expression with relapse-free survival, distant metastasis-free survival and overall survival in breast cancer. (B) Correlations of the catabolic stroma signature with relapse-free survival, distant metastasis-free survival and overall survival in breast cancer. All graphs are calculated using microarray data from 425, 122 and 69 chemotherapy-treated breast cancers, respectively, determined using an online survival analysis tool. Kaplan-Meier correlations are plotted for high (above median, in red) and low (below median, in black) gene expression.
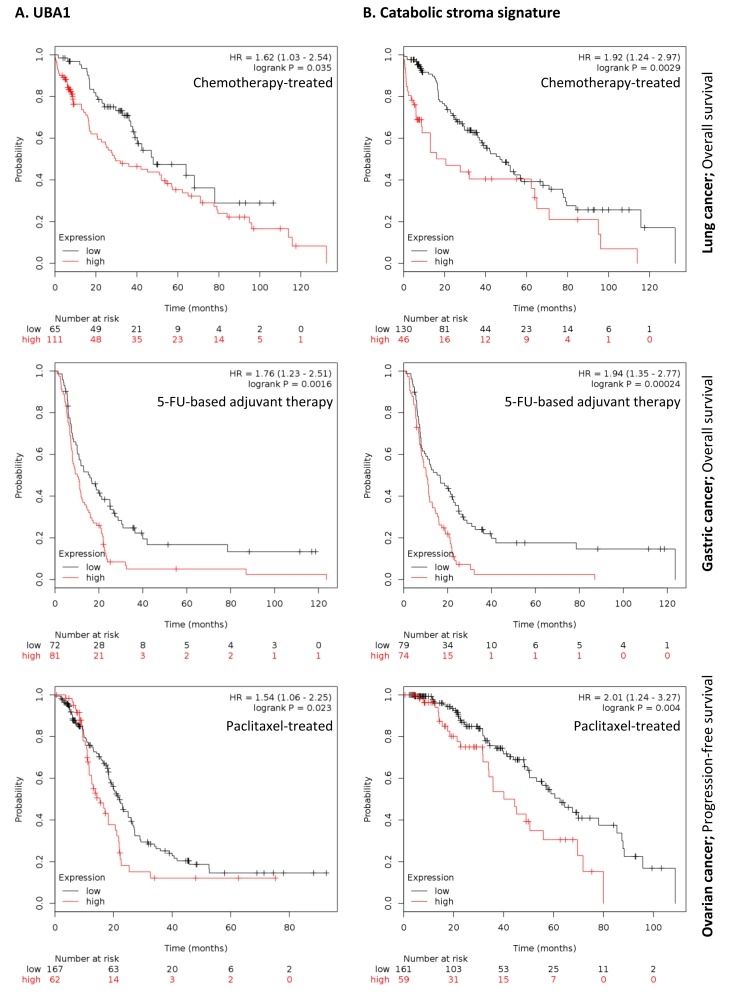
Figure 8. Clinical correlations of UBA1 expression and the UBA1, PSMC5, IGF2R, VAT1, HMOX1, CNN2, TLN1, GNPDA1, G6PD and FASN signature in chemotherapy-treated lung, gastric and ovarian cancer patients. (A) Correlations of UBA1 expression with overall survival in lung and gastric cancer, and with progression-free survival in ovarian cancer. (B) Correlations of the catabolic stroma signature with overall survival in lung and gastric cancer, and with progression-free survival in ovarian cancer. All graphs are calculated using microarray data from 176 chemotherapy-treated lung cancers, 153 5-FU-based adjuvant therapy-treated gastric cancers, and 229 paclitaxel-treated ovarian cancers, determined using an online survival analysis tool. Kaplan-Meier correlations are plotted for high (above median, in red) and low (below median, in black) gene expression.
A taxol-induced catabolic stroma signature was created comprising UBA1, and other proteins representative of the catabolic CAF-like phenotype, including PSMC5, and VAT1, involved in catabolism and vesicle trafficking, several metabolic enzymes including FASN, G6PD and GNPDA, an autophagy marker, IGF2R, the oxidoreductase HMOX1, and myofibroblastic markers including CNN2 and TLN1. See Table 6 for details and abundances of these proteins in taxol-treated fibroblasts. That catabolic stroma signature showed a strong correlation with survival in treated patients (Figure 7B and 8B), whereas that correlation was not seen in non-treated patients (Figure S2B and S3B). Hence, the over-expression of proteins upon treatment with taxol is strongly linked to poor survival, treatment failure and metastasis in breast, lung, gastric and ovarian cancers. Interestingly, most markers used for the signature are also proteins found to be significantly up-regulated in azathioprine-treated hTERT-BJ1 fibroblasts relative to vehicle, including IGF2R, PSMC5, VAT1, HMOX1, CNN2, TLN1 and FASN (Tables 3, 45, S2, S3 and S4). Thus, we conclude that chemotherapy-mediated changes in the abundance of stromal proteins related to the CAF-like catabolic phenotype measured by quantitative proteomics associate with reduced survival, enhanced recurrence and metastasis incidence in several solid malignancies.
Table 6. Proteins of the catabolic stroma signature and their contribution to CAF transformation, metabolism, antioxidant response, autophagy and vesicle trafficking
| CATABOLIC STROMA SIGNATURE | | Taxol | Process |
|---|
| Ubiquitin-like modifier activating enzyme 1 | UBA1 | ↑ 2.36 | Protein degradation |
| 26S protease regulatory subunit 8 | PSMC5 | ↑ 3.17 | Protein degradation |
| Cation-independent mannose-6-phosphate receptor | IGF2R | ↑ 1.88 | Autophagy |
| Synaptic vesicle membrane protein | VAT-1 VAT1 | ↑ 2.44 | Vesicle trafficking / oxidoreductase /inhibits mitochondrial fusion |
| Heme oxygenase (decycling) 1 | HMOX1 | ↑ 2.83 | Antioxidant / oxidoreductase |
| Calponin 2 | CNN2 | ↑ 2.76 | Myofibroblastic differentiation |
| Talin 1 | TLN1 | ↑ 2.95 | Myofibroblastic differentiation |
| Glucosamine-6-phosphate deaminase 1 | GNPDA1 | ↑ 2.46 | Carbohydrate metabolism |
| Glucose-6-phosphate dehydrogenase | G6PD | ↑ 2.69 | Carbohydrate metabolism /generates antioxidant power |
| Fatty acid synthase | FASN | ↑ 1.77 | Fatty acid metabolism |