Forms of IC
Based on the sites of intervention, IC strategies can be classified into two forms, that is, in situ and in remote organs or tissues.
IC in situ
IC in situ involves repeated IR intervention applied locally to the organ or tissue itself, thus offering subsequent protection against IRI-induced lethal injury. The concept of IC in situ was first demonstrated by Murry et al. in 1986, who reported that 4 cycles of 5-min occlusion of the circumflex coronary artery interspersed with 5-min reperfusion could significantly reduce the myocardial infarct size induced by a subsequent longer period occlusion of the same vessel [4]. A systemic review of the role of IC in situ in cardiac surgery summarized data from 22 eligible trials with 933 patients, denoting that local IC might be associated with substantial reductions in ventricular arrhythmia, inotrope requirement, and intensive care unit (ICU) stay [5].
Although direct IC has been discovered for decades and holds the potential in ameliorating IRI, its clinical application is still confronted with great challenges: IC in situ requires a direct intervention employed to the target tissue/organ, which is impractical or even invasive in the cases of IRI of internal organs without surgical issues. Therefore, it would be tempting to discover a way through which an equivalent extent of protection might be brought about without invasive intervention on the targeted organs or tissues.
RIC
RIC refers to a brief, repetitive and sublethal IR applied to an organ or tissue to induce global endogenous tolerance and protect distant (remote) target organs or tissues against subsequent prolonged IRI, meaning that the protective effect of IC can be induced not only in situ directly, but also in distant organs [3].
As reported by previous studies, there are three methods of RIC intervention based on timing of RIC in relation to IRI, including remote ischemic preconditioning (RIPreC, initiated before IRI), remote ischemic perconditioning (RIPerC, initiated at the moment of ischemia) and remote ischemic postconditioning (RIPostC, initiated at reperfusion stage), (Fig. 2) [3].
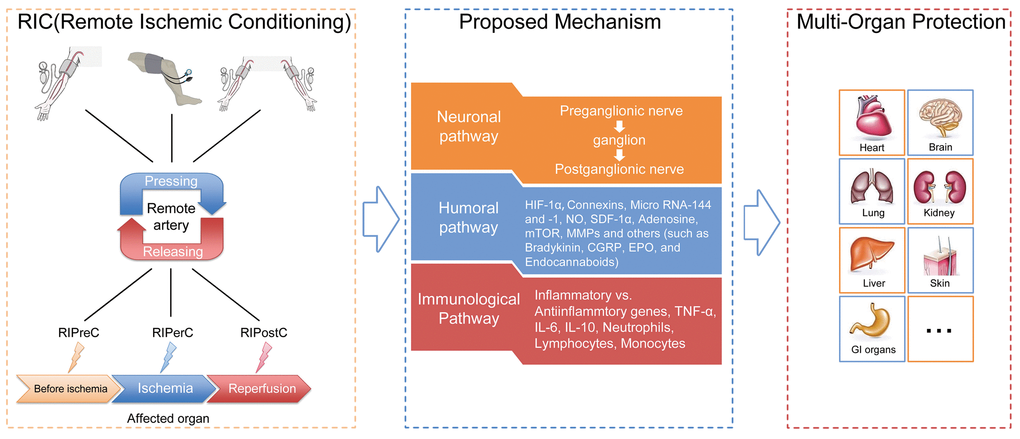
Figure 2. General illustration of remote ischemic conditioning (RIC). Remote ischemic conditioning, in which transient sublethal episodes of ischemia and reperfusion are applied to a limb (upper arm or thigh) or limbs, can be delivered before (remote ischemic preconditioning), during (remote ischemic perconditioning) or after (remote ischemic postconditioning) a subsequent and potentially lethal ischemic attack. Neuronal, humoral as well as immunological mediators are postulated to exert critical roles in the transduction of protective signals generated from limbs and surrounding structures to the targeted organs or tissues. The application of RIC has been extended from initially reducing cardiac infarct sizes resulting from acute myocardial infarction to providing protection for a diversity of organs or tissues (other than the heart), which are likely susceptible to ischemia-reperfusion injury, such as the brain, kidney, lung, liver and skin.
RIPreC. A landmark study for the breakthrough of IC in situ in the possible clinical application was conducted by Przyklenk et al. in 1993, who used 4 cycles of 5-min IR of the circumflex coronary artery prior to the left anterior descending coronary artery (LADCA) occlusion to reduce myocardial infarction volume at the territory of LADCA in a dog model [6]. This protection induced by remote intra-organ preconditioning drew forth the notion of RIPreC and inspired further investigations to clarify this protective effect. Remote inter-organ preconditioning protection was later proved by in a rat model in 1996, showing that a short period of preconditioning forced upon the renal and mesenteric artery reduced the myocardial infarction [7]. A further study using rabbits revealed that a combination of stimulating the gastrocnemius muscle and restricting femoral blood flow could significantly attenuate IRI-induced cardiac injury, indicating the possibility of inducing preconditioning stimulation by using clinically more applicable method such as the limb tourniquet or other occlusion devices [8]. In 2002, Kharbanda et al. carried out two experiments and found that: 3 bouts of 5-min ischemia/reperfusion induced by human upper limb RIPreC could protect the contralateral forearm from endothelial IRI, and 4 bouts of 5-min lower limb RIPreC could elicit a reduction in the extent of myocardial injury against previously sustained infarction in a swine model [9]. These findings facilitated the translation of RIPreC from experimental models to clinical studies. The first successful pilot human study was conducted by Cheung et al. in 2006 in pediatric patients who underwent repair of congenital heart failure [10]. The researchers observed less potential myocardial injury in the RIPreC group as compared with the control group. Subsequent experimental or clinical investigations on RIPreC demonstrated valuable potential of ameliorating IRI in multiple organs.
RIPerC. Another subtype of RIC is RIPerC, which is referred to as the induction of sublethal conditioning stimuli during an episode of ischemic attacks. Schmidt et al. first demonstrated RIPerC by applying cyclical hindlimb IR with a tourniquet in experimental pigs during LADCA occlusion, with a satisfactory result [11]. In two small clinical trials led by Rentoukas and Li, myocardial insults were attenuated among coronary artery disease patients who received RIPerC [12,13]. One classic single-center RCT published in 2010 demonstrated that RIPerC improved myocardial salvage in suspected ST segment elevated myocardial infarction (STEMI) when it was applied during ambulance transport before hospital admission [14].
RIPostC. The history of RIPostC dates back to the first report aiming to investigate the cardioprotection of ischemic postconditioning in 2003, showing that brief repetitive ischemia before fully establishment of flow in a canine model of coronary occlusion-reperfusion could attenuate reperfusion-associated myocardial injury [15]. Two years later in 2005, Staat et al. reported the first clinical trial, that is, postconditioning employed to patients undergoing coronary angioplasty for STEMI might provide protection to hearts [16]. Subsequent publications also demonstrated encouraging outcomes of ischemic postconditioning in patients undergoing cardiac surgeries, as presented by reduced cardiac enzyme levels, morbidity, inotrope scores and the duration of ICU stay [17–19].
Selective meta-analyses published up to December 2014 illustrated the potential salutary effects of postconditioning on cardioprotection in subjects with STEMI scheduled for percutaneous coronary intervention (PCI), although some results might be disappointing or even contradictory [20–22]. Unfortunately, the largest clinical trial so far (DANAMI-3–iPOST) concluded that 4 cycles of IC (30-second balloon occlusion followed by 30-second perfusion) during PCI failed to reduce the primary composite outcome of all-cause death and hospitalization due to heart failure in patients with STEMI [23]. One explanation for this neutral result is the algorithm of IC, which differed from previously reported one (4 cycles of 60-second of occlusion followed by 60-second of reperfusion).
Proposed mechanism of action
The protective mechanisms of RIC through which the protection is transferred to remote targeted organs against IRI are quite sophisticated and have not been fully explored. Current data reveal that the possible underlying mechanisms are correlated with several aspects, mainly including neuronal and humoral pathways, both of which also display inseparable interactions with each other, and the dominant one may depend on the applied stimuli and specific circumstances [29]. Although most observations have thus far been derived from preclinical animal studies focusing with cardioprotection, they might shed light on the exploration of RIC mechanisms in humans. Here below, a precise overview pertaining to some relatively important pathways and associated mediators are recapitulated, (Fig. 2 and Fig. 3).
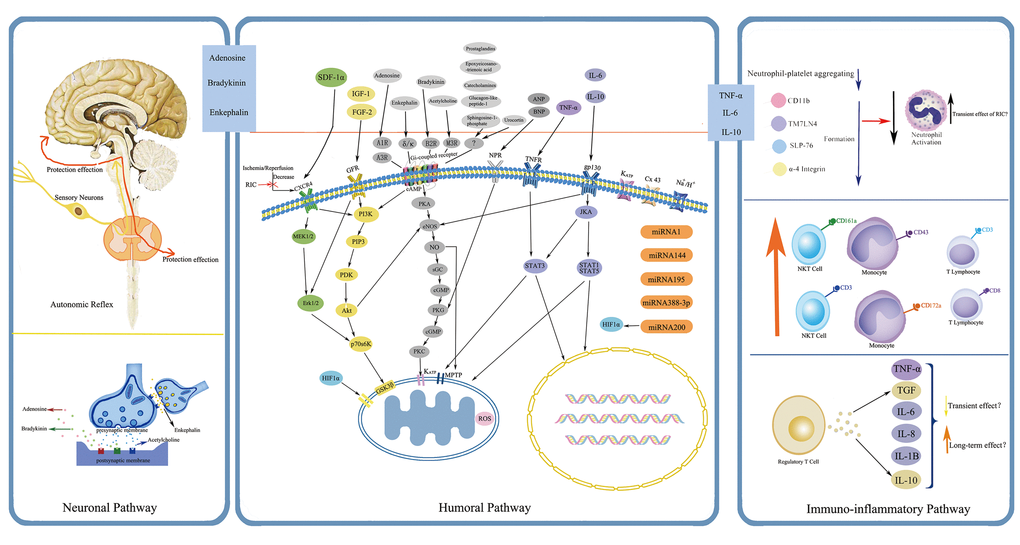
Figure 3. Simplified overview of the protective pathways of Remote Ischemic Conditioning (RIC). eNOS/PKG pathway is presented in gray, RISK pathway in yellow and green, and SAFE pathway in purple. Abbreviations: RISK, reperfusion injury salvage kinase; SAFE, survivor activating factor enhancement; SDF-1α, stromal cell derived factor-1α; IGF-1, insulin like growth factor-1; FGF-2: fibroblast growth factor-2; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-10, interleukin-10; CXCR4, chemokine 4 receptor; GFR, growth factor receptor; A1R, A3R, adenosine receptor A1, A3; δ/κ, δ- and κ- opioid receptor; B2R, bradykinin receptor B2; M3R, muscarinic receptor M3; NPR, natriuretic peptide receptor; TNFR, tumor necrosis factor receptor; gp130, glycoprotein 130; KATP, ATP-dependent potassium channel; Cx 43, connexin43; MEK1/2, also known as mitogen-activated protein kinase kinase 1/2; Erk1/2, extracellular-regulated kinases 1/2; PI3K, phosphatidylinositol-4, 5-bisphosphate3-kinase; PIP3, phos-phatidylinositol-3, 4, 5-biphosphate; PDK, phosphatidylinositol kinase; Akt, also known as protein kinase B; P70s6K, p70 ribosomal protein s6 kinase; GSK3β, glycogen synthase kinase 3β; HIF-1α, hypoxia inducible factor-1α; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; sGC, soluble guanylate cyclase; cGMP, cyclic guanine monophosphate; PKG, protein kinase G; PKC, protein kinase C; MPTP, mitochondrial permeability transition pore; JKA, Janus kinase; STAT1, STAT3, STAT5, signal transducer and activator of transcription 1, 3, 5; ROS, reactive oxygen species; miRNA, microRNA.
Neuronal pathway
The neuronal mechanism has been proposed to play a requisite role in RIC, whereby protective signals from the periphery limbs can be transferred to ischemic organs. A growing body of evidence supports this neuronal hypothetic notion of RIC. For instance, the use of ganglionic blockers such as hexamethonium and trimetaphan could abrogate the RIC-mediated protection in rat models or humans [7,27,34]. Cardioprotection induced by ischemic preconditioning in remote vessels, such as the femoral, renal, and mesenteric arteries has been confirmed in experimental studies, while subsequent transection of the corresponding nerves was able to blunt this effect, suggesting the necessity of afferent nerves for protective signaling transduction [35,36]. In addition, pretreatment with afferent nerve blocker-capsaicin attenuated or even reversed the beneficial effect of RIC in several models, including cerebral, gastric and intestinal ischemic models [37–39]. Moreover, spinal cord reflexes might also exert essential functions in the neuronal pathways, with the supporting evidence that transection of the spinal cord at T9-T10 level and selective blockage of the spinal opioid receptor with naloxone methiodide abolished the RIC effect [35,40]. With regard to the efferent outflow, cardiac studies suggested the crucial role of the parasympathetic nervous system for cardioprotection provided by RIC [41,42]. Although there is still a lack of evidence regarding cerebrovascular diseases, experimental studies in the rat stroke model demonstrated that stimulation of parasympathetic nerve, especially the vagus nerve, could exert a neuroprotective function [43,44]. The function of parasympathetic outflow in RIC was also demonstrated in a human trial by Enko et al. [45].
Humoral pathway
Humoral mediators. The humoral pathway hypothesis refers to repeated cycles of IC at a distant site (for example at limbs) may stimulate the release of certain substances that travel into the blood circulation and then reach the prolonged ischemic organs/tissues to produce a protective effect [46]. This hypothesis was firstly highlighted by a study, which found that the cardioprotective effect of ischemic preconditioning could be transferred from preconditioned to non-preconditioned naïve rabbits through whole blood transfusion [47]. In a porcine transplant model, a study group preconditioned the recipient pigs with limb RIC before receiving hearts from brain-dead donors and ultimately observed a 57% reduction in postoperative cardiac infarct lesions, suggesting that some sort of circulating factors after RIC probably mediated the cardioprotection, even in the absence of intact neural innervation (denervated transplanted hearts) [48]. Shimizu et al. later performed several experiments in order to investigate the humoral nature of limb RIC and its effects on isolated rabbit heart models [11]. The results showed that transferring plasma and dialysate (with 15 kDa dialysis membrane) harvested from donor rabbits undergoing limb-RIC conferred protection to non-preconditioned animals; nonetheless, this phenomenon vanished in subjects who were pretreated with naloxone, implying the possible participation of the opioid receptor pathway. Afterwards, experimental studies, specifically those adopting Langendorff bioassays, proposed that this protection was attributed to unidentified hydrophobic, thermolabile circulating proteins with molecular weights ranging from 3.5 to 15-30 kDa [49,50]. Notably, the presence of protective factors after RIC has also been explored in humans, denoting the results as follows: plasma dialysate from diabetic patients without periphery neuropathy who underwent RIC could confer a cardioprotective effect to rabbit hearts suffering IRI, whereas, this effect was suppressed in hearts immersed with RIC dialysate from diabetic patients concurrent with peripheral neuropathy [51]. A recent study reported that total subdiaphragmatic vagotomy, gastric vagotomy, and selective sectioning of the posterior gastric branch abrogated RIC cardioprotection, suggesting the potentially crucial role of the posterior gastric branch of the vagus nerve in the innervation of circulating factors elicited after RIC [52]. Taken together, RIC might promote the release of some sort of or multiple circulating protective factors that are transferrable between species; an intact neural pathway might be necessarily required for RIC-induced protection; and an tight interaction may exist between the neural and humoral pathways, though details need to be further elucidated.
Despite the clinical importance, actual identification of humoral factors following conditioning response faces great challenges and remains ambiguous. Many proteomic analyses using plasma harvested from both animals and humans undergoing RIC have yielded inconsistent or even paradoxical results. A variety of circulating factors have been proposed as contributors, such as hypoxia inducible factor-1α [53–60], connexin 43 [61–64], microRNA [65–75], nitric oxide [76–82], stromal derived factor-1α [83–86], mammalian target of rapamycin [87–89], matrix metalloproteinases [90–94], adenosine [95–104], bradykinin [105–108], erythropoietin [109], endocannabinoids [110], kallikrein [23], and neuroglobin [111], (Fig. 2). Before being put into clinical application, these potential biomarkers should be reassessed and validated with respect to their efficiency, possible mechanism of action, the changing mode of genomics and proteomics following different conditioning regimens, the time interval that can be detected, and compatibility with RIC-mediated protection, all of which are intriguing but full of challenges.
Immuno-inflammatory responses. Emerging evidence plunks for the fact that induction of endogenous protection via RIC is partially attributed to the modulation of immuno-inflammatory responses. Konstantinov et al. initially discovered that RIC in healthy humans was capable of downregulating proinflammatory genes while upregulating anti-inflammatory genes, around 30 of which were supposed to be involved in leucocyte adhesion, chemotaxis, cell adherence/migration, apoptosis, TNF-α signaling pathway, and Toll-like receptor pathway [112]. Of particular interest, transient unilateral hindlimb ischemia induced in mice could modify myocardial gene expression in response to oxidative stress, inflammation and mitochondrial function at both early and later phases following the procedure [113]. On the one hand, the expressions of proinflammatory genes such as Egr-1 and Dusp 1/6 were decreased. On the other hand, genes involved in the attenuation of oxidative stress response such as HADHSC and peroxiredoxin-4 were strengthened, and those with the potential of aggravating oxidative injury like PDGFRB and Erp57 were suppressed. Particularly, RIC-mediated protection was reflected by reduced neutrophil activation and leukocyte-endothelium interactions [114,115]. However, Albrecht et al. attributed the cardioprotection of RIC to increased infiltration of neutrophils [59]. Similarly, contradictory results were also reported, saying that TNF-α was increased in some RIC models and reduced in others [59,103,115,116]. It may be difficult to explain these seemingly discrepancies about the inflammatory mediators in RIC studies at present in that either neutrophils or TNF-α has been implicated in providing both protective and detrimental effects [117,118]. Additionally, it may be due to limited time points and different experimental subjects or protocols designed for detecting these mediators. The interactions among diverse signal cascades underlying RIC are still obscure. Although IL-6 is well known as a member of the proinflammatory family, ischemic preconditioning has been shown to enhance IL-6 expression and inhibition of IL-6 could attenuate the early preconditioning effect [119,120]. Importantly, elevation in IL-6 is to some extent responsible for the RIC-mediated cardioprotective effects [121]. Cai et al. demonstrated for the first time that IL-10 upregulation promoted late protection by RIC, possibly through the Stat3 signaling pathway [122]. A more recent study investigated the effects of RIC on certain inflammatory/anti-inflammatory mediator profiles and immune cells, together with the mechanism underlying RIC-mediated neuroprotection [123]. They found that RIC ameliorated the post-stroke reduction of peripheral blood CD3(+)CD8(+) T cells and CD3(+)/CD161a(+) NKT cells markedly, and robustly increased the percentage of CD43(+)/CD172a(+) non-inflammatory monocytes. RIC alone without subsequent stroke obviously promoted plasma IL-6 expression without any impact on the concentration of IL-10 and TNF-α. Interestingly, elevated TNF-α expression and further enhanced IL-6 levels were reported in stroke rats subjected to RIC pretreatment. These findings suggest that changes in immune cells populations and cytokines in circulation may be one mechanism contributing to the RIC-mediated neuroprotection. Moreover, it was also demonstrated that the spleen might play a critical role in RIC-mediated alterations in the peripheral immune system and immunomodulation of the splenic response by RIC might create a favorable immune milieu that affects the progression of stroke [124]. However, scant studies have focused on this area, although more work is on the way.
Anti-oxidative stress
An increasing number of researchers have explored the association between RIC and anti-oxidative activity, suggesting that limb RIC could markedly attenuate IRI through downregulating the expression of free radicals while upregulating the expression of antioxidant proteins. For instance, the level of malondialdehyde (MDA), which is frequently used as an indicator to assess the extent of oxidative stress, was significantly reduced following the application of RIC in combination with or without local IC in different animal models, such as cerebral, myocardial, hepatic and renal ischemic models [125–128]. In addition, RIC showed early promise as a protective approach during primary PCI in STEMI patients to enhance the antioxidant potential (increased levels of glutathione peroxidase, superoxide dismutase and total antioxidant capacity) and suppress the increased MDA level [129].
Autophagy
Recently, several investigations have reported that the neuroprotective effects afforded by RIC are closely associated with autophagy activation and the attenuation of mitochondrial injury following cerebral IRI in rat or mice model [130–133]. These findings imply that autophagy exerts a pivotal function in RIC-induced neuroprotection with the possible involvement of AKT/GSK3β, AMPK, and mTOR/p70S6K signaling pathways, and AKT-dependent Bcl-2 phosphorylation. However, in contrast, studies also demonstrated that inhibition of the autophagy process contributed to the protection against cerebral ischemia injury in rats subjected with ischemic post-conditioning alone or combined with RIC [134,135]. The potential function of autophagy in RIC-induced protection needs to be further elucidated.
Improvement of endothelial function and vascular remodeling
Endothelial dysfunction is a common finding in patients with atherosclerotic artery disease, and associated with unfavorable clinical outcomes [136,137]. Studies have shown that repeated RIC stimulus could improve endothelial function in healthy individuals [138,139]. More importantly, in patients undergoing invasive coronary angiography for stable coronary artery disease, long-term, regular RIC has been found to produce improvements in both peripheral and coronary artery function [140,141]. The enhanced endothelial function may be secondary to circulating mediators activated by RIC, such as an increase in endothelial progenitor cells and/or vascular endothelial growth factor (VEGF). Promoting endothelial cells may release tPA and lower the level of PAI in circulation [142]. Other explanations such as reduced oxidative stress and inflammation, and upregulation of endothelial NOS should be considered as well.
Experimental and our pilot clinical studies reveal that chronic RIC is capable of robustly improving cerebral blood flow in chronic cerebral ischemia [143–145]. This amelioration is likely by promoting cerebral angiogenesis, vascular remodeling and collateral formation, as indicated by increased expression of CD31, α-SMA, and VEGF-receptor, as well as the increase in vessels number and volume (3D visualization of cerebrovasculature).
Specific circulating mediators for each organ
Although RIC strategies in different organs/tissues may recruit similar signaling transduction molecules, variations are still recognized, (Fig. 4). Clarifying the role of specific trigger factors for the organ of interest could spur further research in this area and facilitate clinical implement and pharmacological advancement.
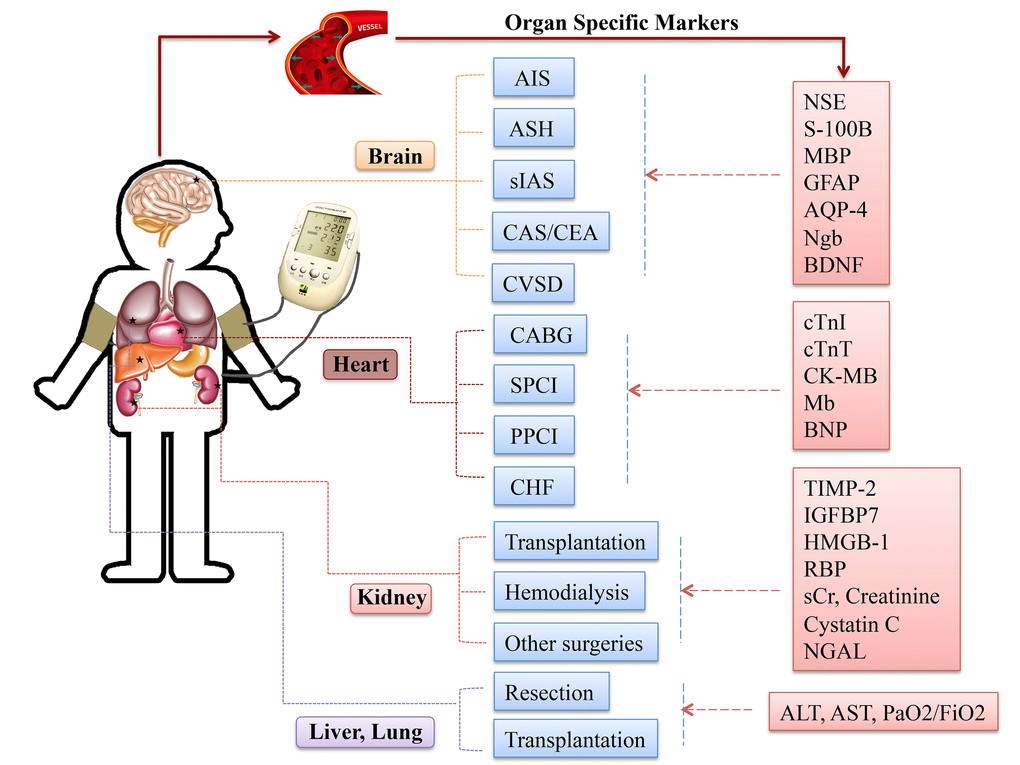
Figure 4. Specific circulating markers for different organ scenarios. Abbreviations: AIS, acute ischemic stroke; ASH, acute subarachnoid hemorrhage; sIAS, symptomatic intracranial arterial stenosis; CEA, carotid endarterectomy; CAS, carotid angioplasty and stenting; CVSD, cerebral small vessel disease; CABG, coronary artery bypass graft surgery; SPCI, selective percutaneous coronary intervention; PPCI, primary percutaneous coronary intervention; CHF, chronic heart failure; NSE, neuronal specific enolase; S-100B, S100 calcium binding protein B; GFAP, glial fibrillary acidic protein; AQP-4, aquaporin-4; Ngb, Neuroglobin; cTnI, cardiac troponin I; cTnT, cardiac troponin T; CK-MB, creatine kinase-myocardial band; Mb, myoglobin; BNP, brain natriuretic protein; TIMP-2, tissue inhibitor of metalloproteinases-2; IGFBP-7, insulin like growth factor-binding protein-7; HMGB-1, high-mobility group box protein-1; sCr, serum creatinine; NGAL, neutrophil gelatinase-associated lipocalin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen.
Current clinical evidence of multi-organ protection
A growing number of clinical trials have implied that RIC is a promising approach to enable multi-organ protection, even though a few studies displayed inconsistent results. Details are shown in Fig. 5 and 6, Supplemental Fig. 1and Supplemental Table 1 to 4.
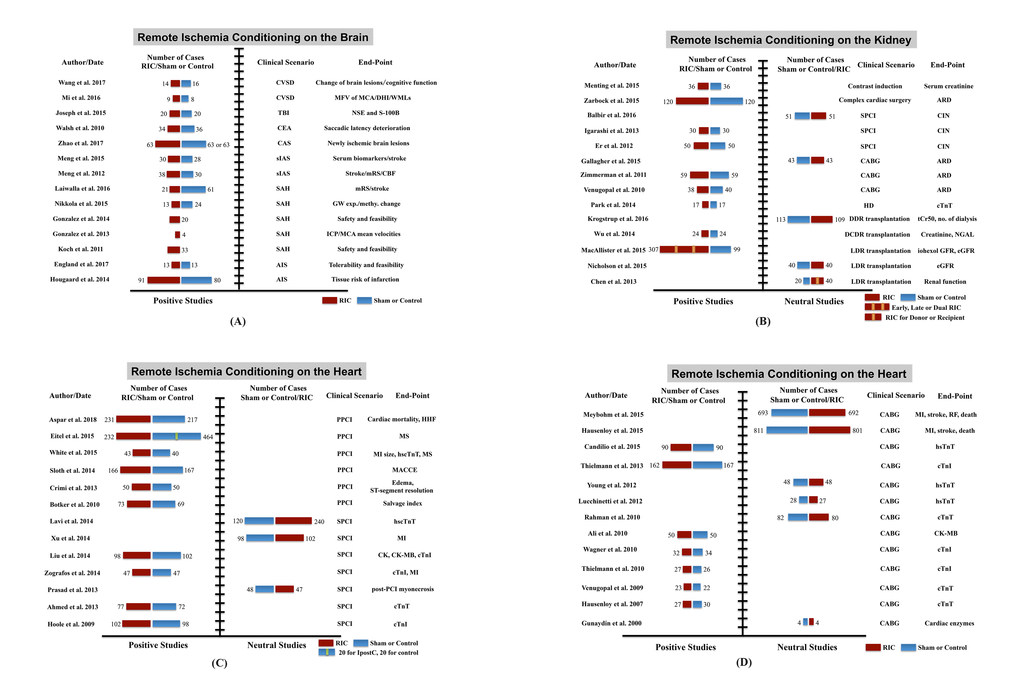
Figure 5. Clinical trials of remote ischemic conditioning (RIC) on multi-organ protection. (A) Studies of RIC effects on the brain, (B) studies of RIC effects on the kidney, (C) and (D) studies of RIC effects on the heart. Abbreviations: CSVD, cerebral small vessel disease; TBI, traumatic brain injury; CEA, carotid endarterectomy; CAS, carotid angioplasty and stenting; sIAS, symptomatic intracranial arterial stenosis; SAH, subarachnoid hemorrhage; AIS, acute ischemic stroke; MFV, mean flow velocity; MCA, middle cerebral artery; DHI, dizziness handicap inventory; WMLs, white matter lesions; NSE, neuron-specific enolase; S-100B, S100 calcium binding protein B; mRS, modified Rankin scale; CBF, cerebral blood flow; GW exp. and methy. change, genome-wide expression and methylation change; ICP, intracranial pressure; SPCI, selective percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; HD, hemodialysis; DDR, deceased donor renal; DCDR, donation after cardiac death renal; LDR, living-donor renal; ARD, acute renal dysfunction; CIN, contrast-induced nephropathy; tCr50, the estimated time to a 50% decrease in baseline plasma creatinine; NGAL, neutrophil gelatinase-associated lipocalin; eGFR, estimated glomerular filtration rate; PPCI, primary percutaneous coronary intervention; HHF, hospitalization for heart failure; MS, myocardial salvage; MI, myocardial infarction; hs-cTnT, high sensitive-cardiac troponin T; hs-cTnI, high sensitive-cardiac troponin I; MACCE, major adverse cardiac and cerebral event; CK, creatine kinase; CK-MB, creatine kinase-myocardial band; RF, renal failure.
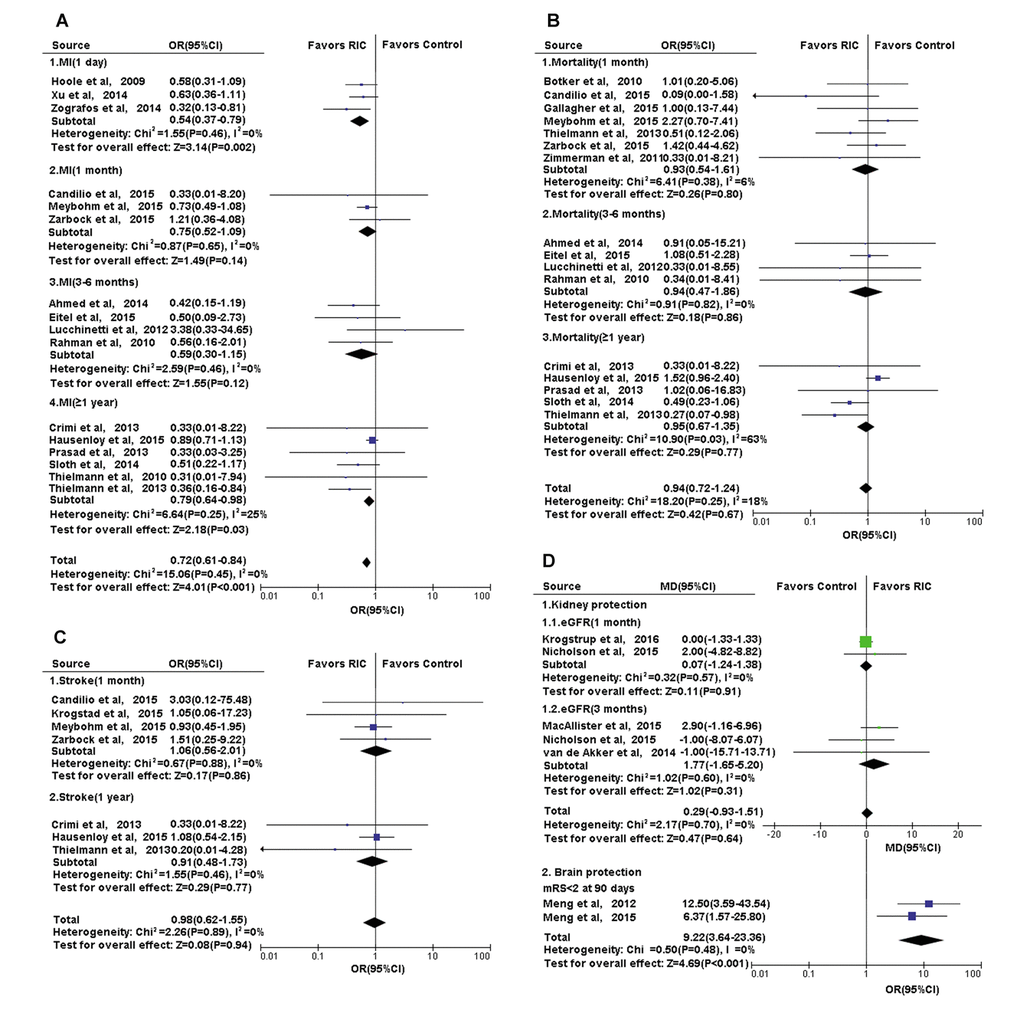
Figure 6. Forest plot with 95% confidence interval for primary or secondary outcomes. (A) Heart: the incidence of complication MI in recipients who underwent CABG or PCI in RIC group compared with controls; the post-treatment mRS in cerebral infarction treated with RIC compared with controls; (B) Heart: the mortality of recipients who underwent CABG or PCI in RIC group compared with controls; (C) Heart: the incidence of complication-stroke in recipients who underwent CABG or PCI in RIC group compared with controls; (D) Kidney and Brain: the eGFR in recipients who underwent renal transplantation and the post-treatment mRS in cerebral infarction, in RIC group compared with controls. Abbreviations: MI, myocardial infarction; CABG, coronary artery bypass graft surgery; PCI, percutaneous coronary intervention; RIC, remote ischemic conditioning; eGFR, estimated glomerular filtration rate; mRs, modified Rankin scale.
Evidence collected so far with regard to the scenarios of target organs are listed below.
Brain
(1) Acute ischemic stroke [146,147], RIC treatment after acute stroke was likely to lower the risk of tissue infarction after 1 month, and improve the neurological outcome as depicted by a reduction in day 90 median National Institutes of Health Stroke Scale (NIHSS) score; (2) chronic cerebral ischemia [142,143,148,149], daily use of bilateral upper limb RIC was safe, tolerable, and able to reduce the recurrence of stroke/transient ischemic attack (TIA), improve cerebral perfusion as well as ameliorate the processes of inflammation, coagulation and fibrinolysis; (3) subarachnoid hemorrhage [150–155], the feasibility and tolerability of RIC were denoted in several proof-of-concept trials; (4) carotid endarterectomy and stenting (CEA and CAS) [156,157], a trend toward fewer saccadic latency deteriorations and a significant reduction in the incidence of new DWI lesions were observed among patients who received RIC; (5) cerebral small-vessel disease [158–160], RIC appeared to be effective in retarding cognition decline and decreasing white matter lesions; and (6) traumatic brain injury [161], it was found that RIC markedly reduced the serum levels of neuron-specific enolase (NSE) and S-100β.
Heart
(1) Selective coronary artery bypass surgery (CABG) [140,162–175], the beneficial effect of RIC in patients undergoing CABG surgery has been reported in several proof-of-concept trials, whereas, two recent large prospective, multicenter, double-blinded, randomized, controlled clinical trials yielded negative results, denoting that RIC failed to influence the incidence of major cardiac and cerebral adverse events, myocardial or renal injury biomarkers and quality of life; (2) selective PCI [176–191], the effect of RIC on patients undergoing selective PCI remains uncertain with discrepant results, and a large-scale, multicenter clinical trial is currently ongoing to explore and verify the potential benefits; (3) primary PCI for STEMI [12,14,192–198], data available are in favor of the RIC-mediated advantageous effect, though larger, well-designed and adequately powered multicenter studies are required to confirm the result; and (4) chronic heart failure [199–201], RIC twice daily for a week ameliorated coronary microcirculation in patients with chronic heart failure without eliciting any adverse effects; RIC once daily for four weeks may enhance skeletal muscle function and lower blood pressure in a compensated state of heart failure, while a general improvement of cardiac function was only seen in most severely compromised patients with chronic heart failure; six-week of twice a day RIC could improve cardiac function in patients with stable heart failure.
Kidney
(1) Renal dysfunction after elective coronary revascularization [202–211], in general, data available showed that RIC decreased the acute renal dysfunction incidence in PCI patients but not in CABG, and the overall incidence of renal replacement therapy and mortality reported in CABG subjects were insignificantly rendered by RIC; (2) high risk for acute kidney injury in the setting of cardiac surgery [207,212], RIC markedly reduced the rate of acute kidney injury within the first 3 days after cardiac surgery, and the 3-month incidence of composite major adverse kidney events, including mortality, renal replacement therapy requirement, and renal dysfunction; (3) renal transplantation [213–218], the application of RIC in the kidney transplantation field yielded conflicting results, most of which were neutral; and (4) dialysis-related ischemic injury [219], only one small pilot study demonstrated that RIC prior to each dialysis practice for a month robustly downregulated the cTnT level (at 1 week and 4 weeks) among patients receiving chronic dialysis.
Lung
(1) Pulmonary dysfunction following cardiac surgeries [10,220,221], no significant difference was found in the pulmonary variables such as postoperative partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) and transpulmonary gradient of inflammatory mediators between the RIC and the control groups, whereas, the incidence of acute lung injury seemed to be reduced by RIC; (2) pulmonary dysfunction following repair of abdominal aortic aneurysm [222], pulmonary dysfunction was robustly attenuated in the RIC patients (higher arterial-alveolar oxygen tension ratio and lower severity of the pulmonary injury score); (3) lung resection for non-small-cell lung cancer [223,224], RIC may afford protection against pulmonary injury as presented by improved intraoperative oxygenation accompanied with the reduced postoperative incidence of acute lung injury; and (4) primary graft dysfunction in the setting of lung transplantation [225], encouraging outcomes were noticed as presented by trends toward a higher level of PaO2/ FiO2, a lower primary graft dysfunction severity score and a reduced incidence of rejection in RIC treated patients.
Liver
(1) Liver injury after cardiac valve replacement surgery [221], the levels of total bilirubin, instead of serum transaminases and albumin, were lower in the RIC group; (2) major liver resection for colorectal liver metastasis [226], RIC significantly ameliorated liver injury after hepatectomy as presented by decreased serum transaminases and higher indocyanine green clearance; and (3) liver transplantation [227,228], a single center, randomized clinical trial aiming to elucidate RIC-mediated protection in liver transplant recipients is underway.
Gastrointestinal Organs
(1) Intestinal injury after open infrarenal abdominal aortic aneurysm repair surgery [222], one small randomized trial assessed the potential use of RIC in GI protection, illustrating that transient RIC substantially reduced the intestinal injury in patients undergoing open infrarenal abdominal aortic aneurysm repair surgery.
Skin
(1) Cutaneous microcirculation in healthy volunteers [229–231], RIC may have a great potential in improving blood perfusion and alleviating soft tissue injury due to accidental or operative trauma and plastic versus reconstructive surgery.
RIC and ageing
As the average human lifespan has increased markedly over the past decades, the mounting incidence of ageing-related disorders in the elderly, and the related economic and social burden, impel scientists to develop novel strategies to slow down or prevent these disorders, alleviating the suffering at the end of life.
Decreased physiological reserve and tissue resilience are characteristics of biological ageing, which render the human system more susceptible to pathological threats. Given the fact that elderly patients usually have at least two afflicted organs or tissues, therapeutic approaches with systemic actions (inducing protective responses in a wide range of organs and tissues) are warranted. In addition to the efficacy, safety and compliance issues are of great importance in an ageing population. The emerging area of RIC builds upon this foundation. The capability of this non-pharmaceutical and non-surgical intervention to protect vital organs simultaneously by enhancing the body’s powers to adapt to pathological threats could provide a safe, less burdensome, minimally-invasive way for ageing-related disorders. Currently, RIC is being evaluated in a variety of clinical settings such as cerebrovascular disease, coronary artery disease and renal injury that predominantly influence the older population.
Challenges and promising
To date, regarding the safety and tolerability of the methodology, no RIC-associated adverse events have been reported in the published clinical studies. Although the prospect of clinical transformation of RIC on multi-organ protection is promising, challenges still exist. The controversial findings raise the question “Can RIC have impact on clinical outcomes in patients under different clinical conditions?” To the best of our knowledge, a number of factors should be reconsidered thoroughly in the future work.
For instance, although previous experimental work has implied that the number and duration of IR cycles might affect the efficacy of RIC, there is a paucity of clinical data comparing the effectiveness of different RIC protocols, and no convincing evidence of the most favorable conditioning strategy has been established [232]. Notably, the ideal regimen for limb RIC may vary on the basis of clinical scenarios where they work best. Hence, optimizing the RIC regimens for different clinical settings are pivotal issues waiting to be addressed.
Establishment of biomarkers has been considered as a paramount step for translating RIC from preliminary data into real clinical practice. Many hurdles still lie ahead to screen and discover the appropriate set of biomarkers in order to accurately evaluate the clinical efficacy of RIC. The investigations of a wider panel and combination use of genomics, proteomics and imaging markers are warranted. For instance, it was reported that more than 150 genes including apoptosis, cell survival, and immunity-related genes were differentially expressed in healthy subjects who were preconditioned with repetitive arm ischemia [112]. Following this, a pilot study exploring genome-wide expression and methylation changes in patients with aneurysmal subarachnoid hemorrhage revealed that a group of genes in cell cycle, defense, and inflammatory responses were rendered by RIC [154]. Novel techniques such as gene chip, serial analysis of gene expression and genome-wide expression studies may largely facilitate understanding of the conditioning response [114].
Essentially, the potential applications of RIC are numerous and may be extended far more than aforementioned clinical settings. Fox example, RIC has been found to reduce the severity of acute mountain sickness symptoms, enhance the exercise performance and benefit patients with diabetic foot ulcer [233–237]. On an additional note, animal studies have highlighted the potential of limb RIC in diverse retinal disorders such as promoting retinal ganglion cell survival after optic nerve transection and affording protection to retinal photoreceptors against bright light- or IRI-induced photoreceptor degeneration [238–240]. Knowing that the proposed mechanisms of RIC involve modulation of multiple pathways that cover inflammatory response, apoptosis, and oxidative stress, its therapeutic potential is believed to be able to expand to some non-ischemic inflammatory diseases with at least partially similar pathophysiological processes such as inflammatory bowel disease, acute pancreatitis, and connective tissue disorders. In addition, evidence available suggests that long-term RIC is capable of promoting improvement of the vascular function (such as improvement of local and systemic endothelial function, and microcirculation), which may be related to lower risks of future cerebro- and cardiovascular adverse events [138,139]. As the application of RIC in these probable candidates is still in infancy, the feasibility will be determined in more rigorously designed experimental and clinical studies.
There are conceivably a number of existing and potential stakeholders when it comes to the extrapolation of RIC from test bench to bedside. One of the major explanations for these discrepancies lies in the impact of comorbidities on the ischemic tissues or organs in response to the benefits from IC. Animals used in experimental studies are mainly juvenile, genetically homogenous, housed in similar living environments with the same diets, and carried with limited comorbidities. Nevertheless, a significant challenge in clinical trials is that enrolled individuals are more aged with large comorbidities and polypharmacy. In fact, sets of comorbidities including ageing, hypercholesterolemia, hypertension, diabetes, and chronic kidney disease have been found to inhibit or abolish the potency of IC according to previous experimental findings. It seems that concomitant medications including volatile anesthetics such as propofol and isoflurane, opioids, statins, anti-platelets, ACEI, beta-blockers, nitrates and hypoglycemic agents such as pioglitazone and glimepiride might either abrogate or restore the beneficial effects afforded by IC [167,241–246]. One primary concern in treating patients with acute ischemic cerebro- or cardiovascular accident is the inevitable healthcare transport delay, which is believed to be closely associated with unfavorable clinical outcomes. A single center clinical study reported that RIC conducted during transportation to the hospital lessened the prejudicious effect of transport delay on myocardial salvage in STEMI patients undergoing primary PCI, and this cardioprotective effect was more marked in those with extended delay (healthcare system delay > 120 min) [247]. Moreover, the location and severity of infarcts, and the coronary collateral circulation’s ability may also play a potential role for mediating the effect of RIC [248]. A better realization regarding the limitations of current experimental or clinical design together with the way through which comorbidities, medications and other factors affect the efficacy of RIC will facilitate the selection of beneficial population, help understanding the underlying mechanisms and optimize the RIC regimen.
Intriguingly, pharmacological manipulation targeting the signaling cascades and related molecular participants that can modulate the endogenous mechanisms of RIC is an emerging notion with substantial clinical value. Figuring out common mechanisms of protection afforded by IC and some pharmacological agents in a given clinical set-up may facilitate the development of novel approaches against deleterious effects such as IRI. In addition, the presence of time windows during which the signaling cascades in responsive to IRI are engaged might affect not only the effectiveness of IC but medical interventions that target perpetrators of reperfusion injury. Clearly, research should be continue for the sake of identifying pharmacological mimetic candidates that are safe and efficacious, verifying time window for conditioning, and further delineating the mechanism behind as well as human genetic and proteomic responses to conditioning.
Key points
In this study, we provided an overview of the basic concept and proposed protective mechanisms of remote ischemic conditioning (RIC). In addition, we comprehensively reviewed and summarized the clinical studies of RIC in multi-organ protection published so far. Potential obstacles, which may retard the clinical translation of RIC, were discussed as well. We found that:
Proof-of-concept clinical studies demonstrated benefits with RIC as an adjuvant to thrombolysis, mechanical thrombectomy or primary angioplasty in patients with acute cerebro- or cardiovascular attack, such as ischemic stroke and acute myocardial infarction; in light of advantages including simplicity, low expenditure and safety, RIC may be an ideal intervention to be employed during the transportation to qualified stroke or chest pain centers, and before and/or after intrahospital treatment.
Long-term, repeated use of RIC has the potential to improve vascular endothelial function and enhance tissue perfusion, whereby decreasing the incidence or recurrence of unfavorable clinical endpoints in diverse clinical settings, such as chronic cerebral ischemia and chronic heart failure.
Although two large, multicenter, randomized clinical trials of RIC in coronary artery bypass graft surgery yielded negative outcomes, factors including patient selection, RIC protocol and potential confounders should be further explored before reaching a final conclusion.
Regarding the multi-organ protection feature, RIC seems to be a promising non-pharmaceutical and non-surgical approach for preventing and treating systemic vascular diseases, which can inflict multiple systems simultaneously. As multi-organ ischemia-reperfusion injury, particularly amongst elderly patients with clinical scenarios such as atherosclerotic vascular disease, heart surgery and organ transplantation, is one of the most common real-world situations that besets clinicians, the authors believe that this paper will certainly arouse a wide interest from medical workers in both tertiary and community-based medical facilities.
Future experimental or clinical work should focus on addressing the issues that may influence the translation of RIC from test bench to bedside, such as identifying the protective mechanism underlying ischemic conditioning, optimizing the conditioning regimen, establishing biomarkers to accurately evaluate the efficacy of RIC, and figuring out the impact of potential comorbidities, medications and other factors on RIC.