Hallmarks: cancer cell of origin vs. e-CSCs
Cancer cells are believed to originate from senescent cells that have undergone stress-induced cell cycle arrest [1–3]. During chronological aging, genetic mutations are thought to accumulate in the stem cell compartment, resulting in i) oncogene activation, ii) tumor suppressor inactivation, as well as iii) a variety of genetic chromosomal rearrangements (deletions, translocations and duplications) and other epigenetic modifications [4–6].
Presumably, cancer cells may arise from senescent cells through a process of re-activation, leading from cell cycle arrest to hyper-proliferation. It has been proposed that telomerase activity (hTERT) catalyzes this transition [3]. However, this process of re-activation can also be achieved through cellular metabolism [7,8]. For example, David Sinclair and his colleagues at Harvard Medical School have shown that senescent cells suffer from a deficiency of NADH (nicotinamide adenine dinucleotide), a key anti-oxidant, and that senescent cells can be revived simply by the addition of an NADH precursor metabolite (namely, nicotinamide riboside) to the cell culture media [7,8]. These results directly imply that the anti-oxidant response may also trigger the revival of senescent cells (Figure 1). Similarly, cancer stem cells (CSCs) are strictly dependent on NADH, for their propagation as 3D-spheroids [9]. Also, CSCs have been shown to over-express classical “embryonic” markers of stemness, such as Oct4, c-Myc and Nanog, among others.
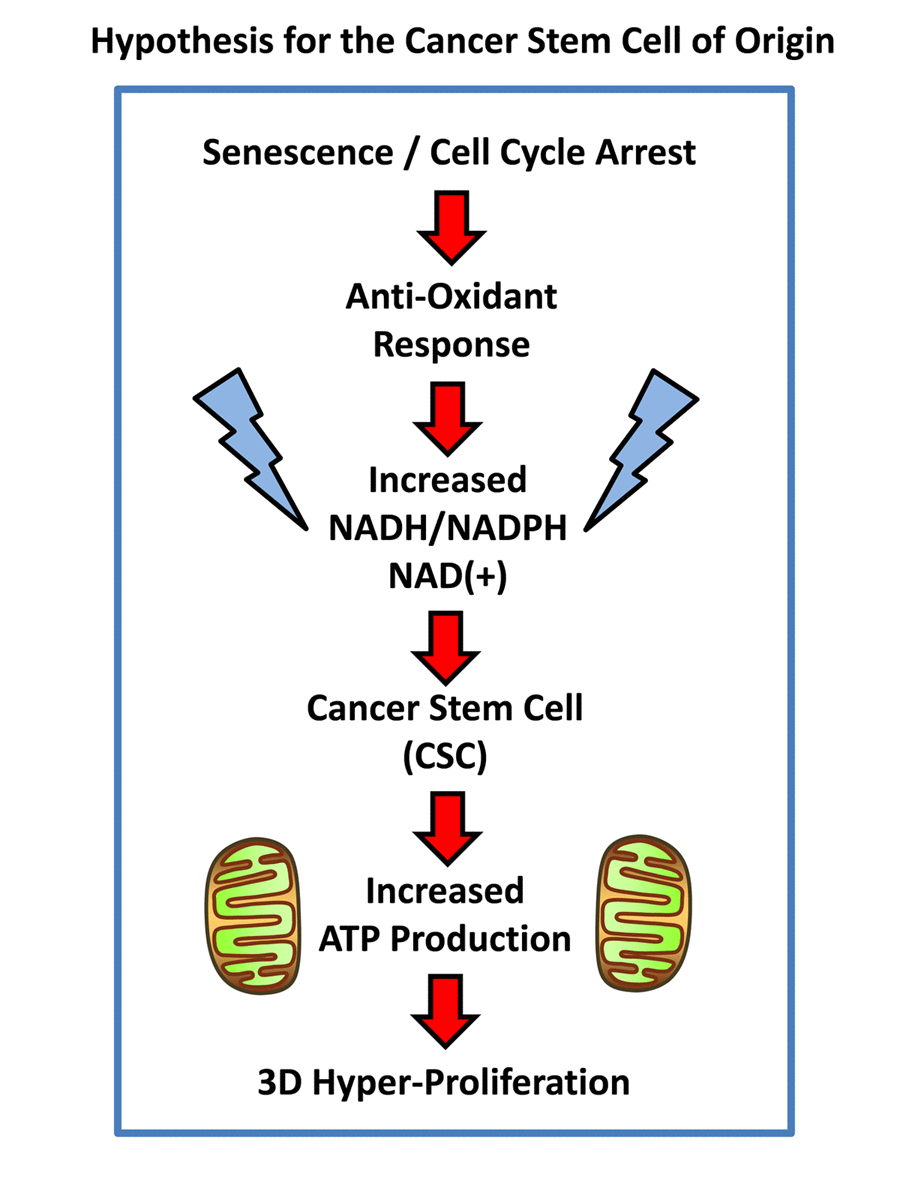
Figure 1. Hypothesis for how senescent cells can mechanistically become cancer stem cells. Senescent cells undergoing cell cycle arrest mount an anti-oxidant defense, to increase their levels of NADH. In turn, increased NADH levels are known to be sufficient to rescue senescent cells from cell cycle arrest, allowing new cell proliferation, by “re-activating” or “resuscitating” senescent cells. Increased mitochondrial power would then drive elevated ATP production and 3D anchorage-independent growth, fostering the generation and propagation of the cancer cell of origin.
Therefore, the cancer stem cell of origin [10,11] would be predicted to retain certain properties of senescent stem cells, while undergoing a gain-of-function process, thereby obtaining new properties of a cancer cell, resulting in a chimeric or hybrid phenotype (Figure 2). These properties would be expected to include: biological markers of senescence; a hyper-proliferative phenotype; a very active metabolic program to support anabolic growth and proliferation; an anti-oxidant response, for driving the revival program, to overcome senescence-induced cell cycle arrest [7,8,12]; and key stem cell features (See Table 1, Left).
Table 1. Expected hallmarks of the cancer cell of origin: Comparison with e-CSCs.
| Cell Type: | Cancer Cell of Origin | e-CSCs |
| Properties: | Expected Features | Observed Features |
| 1) Cell Cycle Arrest: | Senescence Markers | Elevated p21-WAF (~17-fold) |
| 2) Propagation: | Hyper-Proliferative | G0/G1: ~35-37% S-phase: ~10-18% |
| | G2/M: ~32-33% Polyploid: ~12-17% |
| 3) Metabolism: | Metabolically Active | Increased Mitochondrial Mass (~4-fold); High OXPHOS & Glycolysis |
| 4) REDOX: | Anti-Oxidant Response | ALDH Functional Activity (~9-fold Increased) |
| [Produces NADH] | Glutaredoxin-1 (GLRX) (~11-fold Increased) |
| ALDH3A1 (~10-fold Increased) |
| QPRT (~4-fold Increased) |
| RRM2, GCLC, NQO2 (Each ~2-fold Increased) |
| 5) “Stemness”: | Stem Cell Markers | High Flavin-based Auto-fluorescence (FAD/FMN); Large Cell Size; Aldefluor (+); Anchorage-Independence; |
| BCAS1 (+) (>100-fold Increased) |
| 6) Inhibitors: | Unknown | Mitochondrial OXPHOS Inhibitors and CDK4/6 Inhibitors |
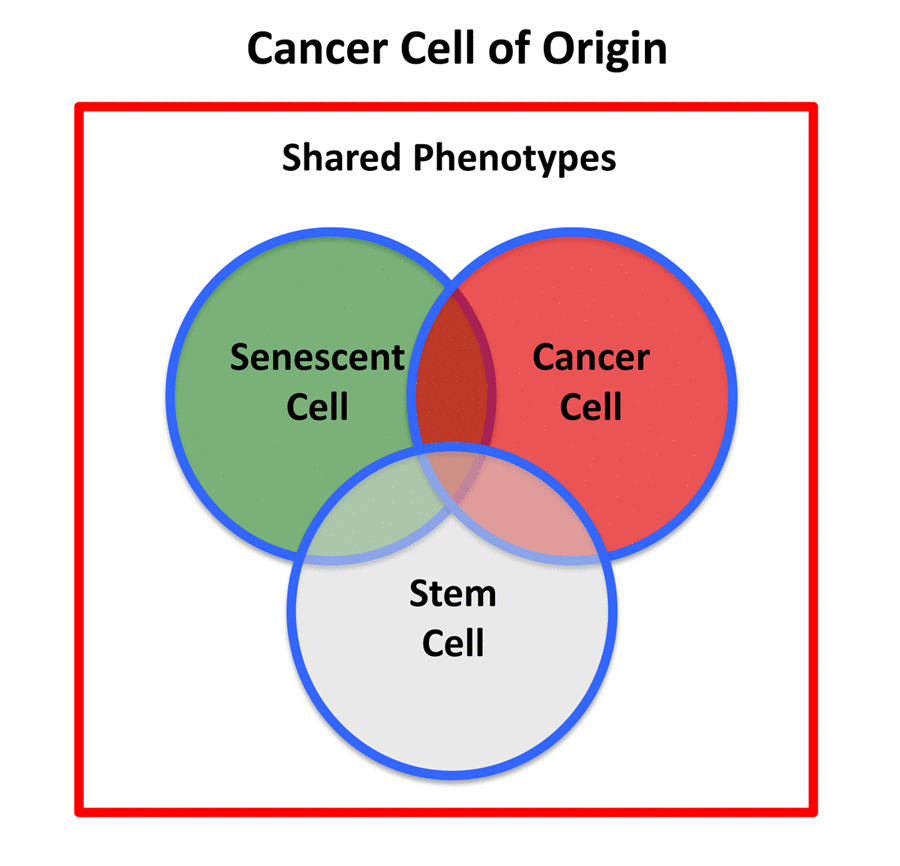
Figure 2. Cancer stem cell of origin. The cancer stem cell of origin would be predicted to have a chimeric- or hybrid-phenotype, retaining elements of i) senescent cells, ii) cancer cells, and iii) stem cells, as we observe in e-CSCs.
Recently, our laboratory may have fortuitously isolated a new tumor cell with a cancer cell of origin phenotype, by using flavin-derived auto-fluorescence as a selection marker, via flow-cytometry [13]. To functionally describe these cells, we coined the term “energetic” cancer stem cells (e-CSCs) [13]. Briefly, e-CSCs retain high expression of the senescence marker p21-WAF (CDKN1A), while paradoxically manifesting a hyper-proliferative phenotype (Table 1, Right). Based on Ingenuity Pathway Analysis (IPA) of e-CSC proteomics data, other upstream regulators of cell cycle arrest and senescence were activated, including p53, TGFB1, and p38 MAPK signaling [13].
Moreover, e-CSCs show a hyper-metabolic phenotype, with increased mitochondrial mass, elevated oxidative mitochondrial metabolism, as well as enhanced glycolytic function. e-CSCs mount a strong anti-oxidant response, characterized by increased levels of glutaredoxin-1 (GLRX) expression and ALDH isoform activity (Table 1, Right). Interestingly, e-CSCs also show a number of stem-like features, including large cell size and anchorage-independent growth, that is highly sensitive to inhibitors of both mitochondrial OXPHOS and CDK4/6 cell cycle progression (Figure 3). In addition, large cell size is a key feature commonly shared by CSCs, e-CSCs and senescent cells alike [13–16].
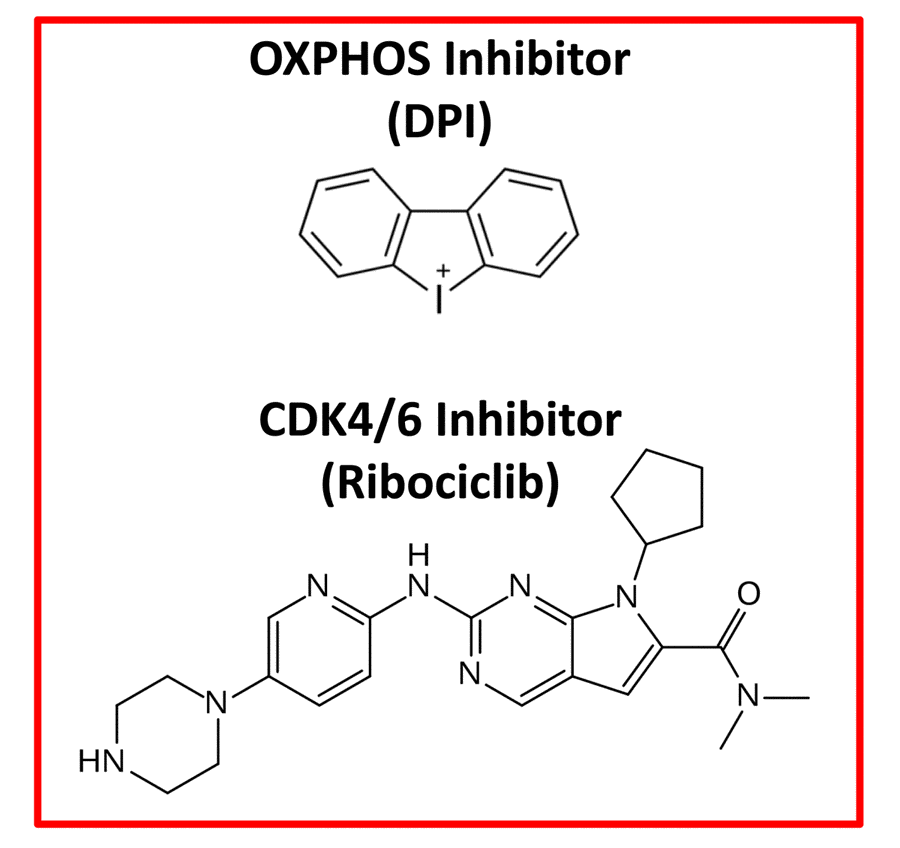
Figure 3. Therapeutic targeting of e-CSCs. Experimentally, both an OXPHOS inhibitor (Diphenyleneiodonium; DPI) and a CDK4/6 (Ribociclib) inhibitor were effective in abrogating the 3D-propagation of e-CSCs.
Lastly, in e-CSCs, BCAS1 (Breast carcinoma-amplified sequence-1) protein expression was up-regulated, by nearly 120-fold [13]. BCAS1 over-expression [17] is functionally associated with “stemness” [18], a more aggressive cancer cell phenotype [19] and Tamoxifen-resistance [20,21]. MCF7-TAMR cells, which were chronically selected for Tamoxifen-resistance, by including Tamoxifen in their tissue culture media, show a >50-fold increase in BCAS1 protein expression, promoting increased ATP production [21].
We are tremendously grateful to Rumana Rafiq, for her kind and dedicated assistance, in keeping the Translational Medicine Laboratory at the University of Salford running very smoothly. We would like to thank the Foxpoint Foundation (Canada) and the Healthy Life Foundation (UK) for their philanthropic donations towards new equipment and infrastructure, in the Translational Medicine Laboratory at the University of Salford.
MPL and FS hold a minority interest in Lunella Biotech, Inc.
This work was supported by research grant funding, provided by Lunella Biotech, Inc (to FS and MPL).