Effects of the antibiotic cocktail on body weight
A repeated measures two-way ANOVA revealed that treatment with an antibiotic cocktail for 14 days reduced mouse body weights (antibiotic: F1,36 = 20.549, P < 0.001; MPTP: F1,36 = 0.005, P = 0.994; interaction (antibiotic × MPTP): F1,36 = 0.045, P = 0.833; Figure 1B). On day 22, antibiotic + saline group body weights, but not antibiotic + MPTP group weights, remained lower than those of mice that did not receive antibiotics (Figure 1B). Thus, antibiotic + MPTP group body weights recovered gradually after treatment, while antibiotic + saline group weights did not.
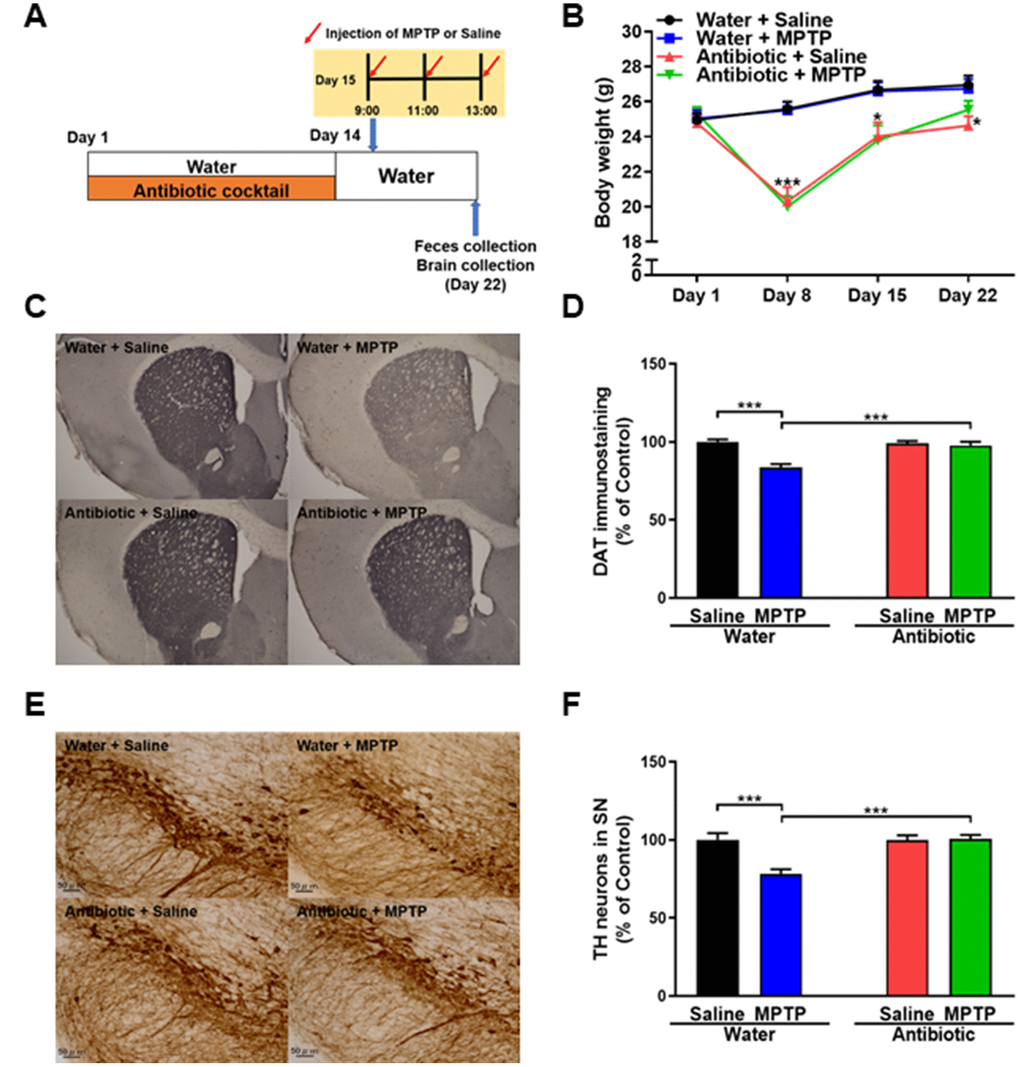
Figure 1. Effects of antibiotic treatment on gut microbiota diversity. (A) Treatment schedule. Adult mice received drinking water with or without antibiotic cocktail from day 1 to day 14. On day 15, MPTP or saline injections were administered. On day 22, fresh feces were collected. Mice were then perfused for immunohistochemistry. (B) Body weights in the different groups (repeated two-way ANOVA, antibiotic: F1,36 = 20.549, P < 0.001; MPTP: F1,36 = 0.005, P = 0.994; interaction (antibiotic × MPTP): F1,36 = 0.045, P = 0.833). (C) Representative images of DAT immunohistochemistry in the water + saline, water + MPTP, antibiotic + saline, and antibiotic + MPTP groups. (D) Striatal DAT immunoreactivity data. (E) Representative images of TH immunohistochemistry in the water + saline, water + MPTP, antibiotic + saline, and antibiotic + MPTP groups. (F) SNr TH immunoreactivity data. Data are shown as mean ± S.E.M. (n = 10). ***P < 0.001. Bar = 50 μm.
Antibiotic treatment protected against MPTP-induced neurotoxicity in the mouse brain
DAT immunohistochemistry revealed that MPTP reduced DAT levels in the striatum of the water-treated group, but not the antibiotic-treated group (Figure 1C). A two-way ANOVA revealed significant differences in DAT immunoreactivity among the four groups (antibiotic: F1,36 = 11.30, P = 0.002; MPTP: F1,36 = 20.46, P < 0.001; interaction (antibiotic × MPTP): F1,36 = 15.32, P < 0.001; Figure 1D). TH immunohistochemistry revealed that MPTP reduced TH immunoreactivity in the SNr of the water-treated group, but not the antibiotic-treated group (Figure 1E). A two-way ANOVA revealed significant differences in TH immunoreactivity among the four groups (antibiotic: F1,36 = 11.48, P = 0.002; MPTP: F1,36 = 10.19, P = 0.003; interaction (antibiotic × MPTP): F1,36 = 11.75, P = 0.002; Figure 1F). Collectively, these results indicate that treatment with an antibiotic cocktail for 14 days protected against MPTP-induced dopaminergic neurotoxicity in the striatum and SNr.
Gut microbiota composition
Next, we investigated the composition of the gut microbiota, which can be altered by antibiotic cocktail treatment [33–35], in the four experimental groups. α-diversity is defined as the richness of gut microbiota and can be measured using different indices. Two-way ANOVAs revealed a significant difference in the Chao1 (antibiotic: F1,36 = 15.928, P < 0.001; MPTP: F1,36 = 37.541, P < 0.001; interaction (antibiotic × MPTP): F1,36 = 20.587, P < 0.001; Figure 2A) and Ace (antibiotic: F1,36 = 12.968, P < 0.001; MPTP: F1,36 = 43.032, P < 0.001; interaction (antibiotic × MPTP): F1,36 = 22.827, P < 0.001; Figure 2B) indices among the four groups. Specifically, Chao 1 and Ace indices were higher in the water + MPTP group than in both the water + saline and antibiotic + MPTP groups (P < 0.001). Interestingly, antibiotic cocktail treatment attenuated the MPTP-induced increase in the Chao 1 and Ace indices. A two-way ANOVA also revealed significant differences in the Shannon index among the four groups (antibiotic: F1,36 = 8.942, P = 0.005; MPTP: F1,36 = 0.593, P = 0.446; interaction (antibiotic × MPTP): F1,36 = 3.427, P = 0.072; Figure 2C). The Shannon index in the antibiotic + MPTP group was lower than that of the water + MPTP group. In an unweighted UniFrac PCoA dot map, dots representing the antibiotic-treated groups were far away from dots representing the water-treated groups (Figure 2D). Interestingly, dots representing the antibiotic + MPTP group were isolated from dots representing the other three groups (Figure 2D).
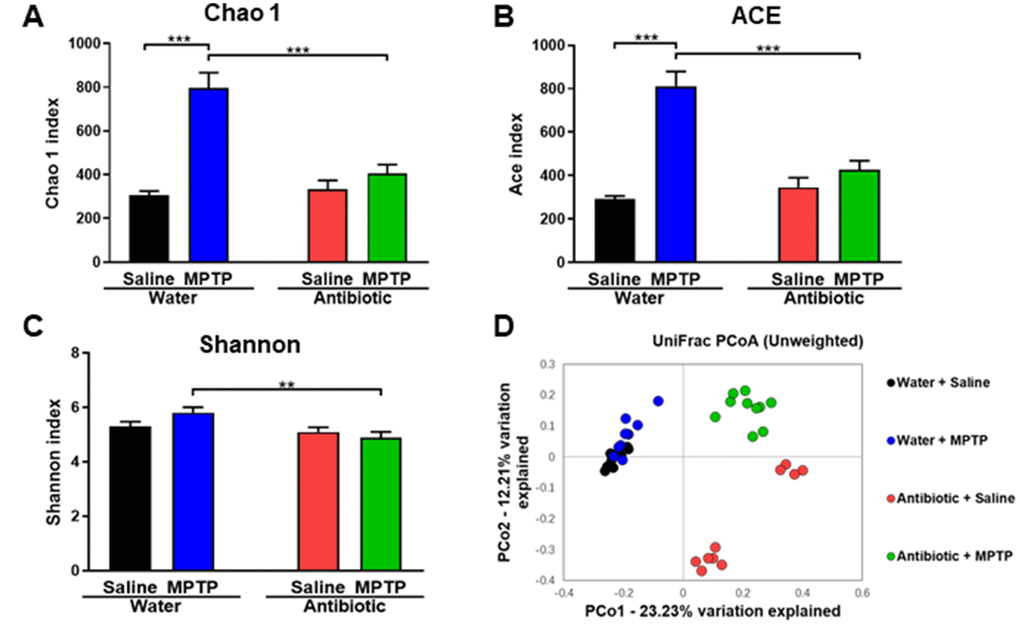
Figure 2. α-diversity and β-diversity in gut microbiota. Diversity index values for the four groups. (A) Chao 1 index (two-way ANOVA, antibiotic: F1,36 = 15.928, P < 0.001; MPTP: F1,36 = 37.541, P < 0.001; interaction (antibiotic × MPTP): F1,36 = 20.587, P < 0.001). (B) ACE index (two-way ANOVA, antibiotic: F1,36 = 12.968, P < 0.001; MPTP: F1,36 = 43.032, P < 0.001; interaction (antibiotic × MPTP): F1,36 = 22.827, P < 0.001). (C) Shannon index (two-way ANOVA, antibiotic: F1,36 = 8.942, P = 0.005; MPTP: F1,36 = 0.593, P = 0.446; interaction (antibiotic × MPTP): F1,36 = 3.427, P = 0.072). (D) PCoA analysis of gut bacteria data (Bray–Curtis dissimilarity).
At the phylum level, Firmicutes were the most abundant phylum in the water + saline group microbiota (Figure 3A and 3B). The abundance of Firmicutes was lower in the antibiotic + MPTP group than in the water + MPTP and antibiotic + saline groups (P < 0.001, Figure 3B). In contrast, the most dominant phylum in the antibiotic + MPTP group, Bacteroidetes, was less abundant in the water + MPTP and antibiotic + saline groups (P < 0.001, Figure 3C). Proteobacteria levels were higher after treatment with the antibiotic cocktail compared to the two water-treated groups (Figure 3D), while Deferribacteres and TM7 levels decreased after treatment with the antibiotic cocktail or with MPTP (Figure 3E, 3F).
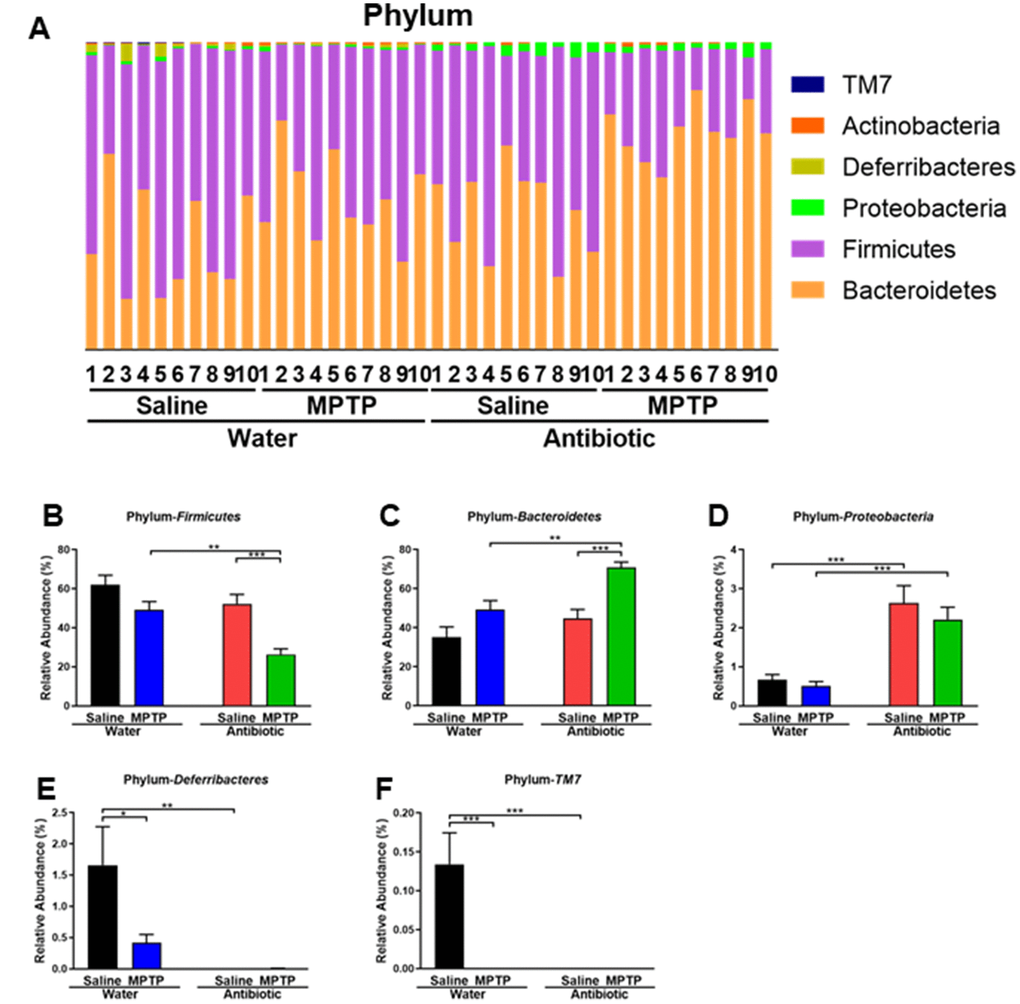
Figure 3. Altered gut bacteria composition at the phylum level. (A) Relative abundance at the phylum level. (B) Bacteroidetes. (C) Firmicutes. (D) Proteobacteria. (E) Deferribacteres. (F) TM7. Data are shown as mean ± S.E.M. (n = 10). **P < 0.01, ***P < 0.001. See the Supplementary Table 1 for detailed statistical analysis.
Antibiotic and MPTP treatment also altered the composition of fecal microbiota at the genus level (Figure 4A). Lactobacillus,Mucispirillum, and Candidatus Arthromitus levels decreased after treatment with the antibiotic cocktail (Figure 4B–4D). In contrast, Parasutterella, Blautia, Robinsoniella, Escherichia, Dorea, and Eubacterium levels increased after treatment with antibiotic cocktail (Figure 4E–4J). Interestingly, Asaccharobacter levels increased in the antibiotic + MPTP group compared to the other three groups (Figure 4K). Clostridium, which was the most dominant genus in control mice, decreased after MPTP injections and antibiotic cocktail treatment (Figure 4L). Furthermore, the antibiotic + MPTP group had a higher abundance of Parabacteroides than the other three groups (Figure 4M). Finally, MPTP treatment attenuated the antibiotic-induced increase in the abundance of Bacteroides and Enterococcus (Figure 4N, 4O).
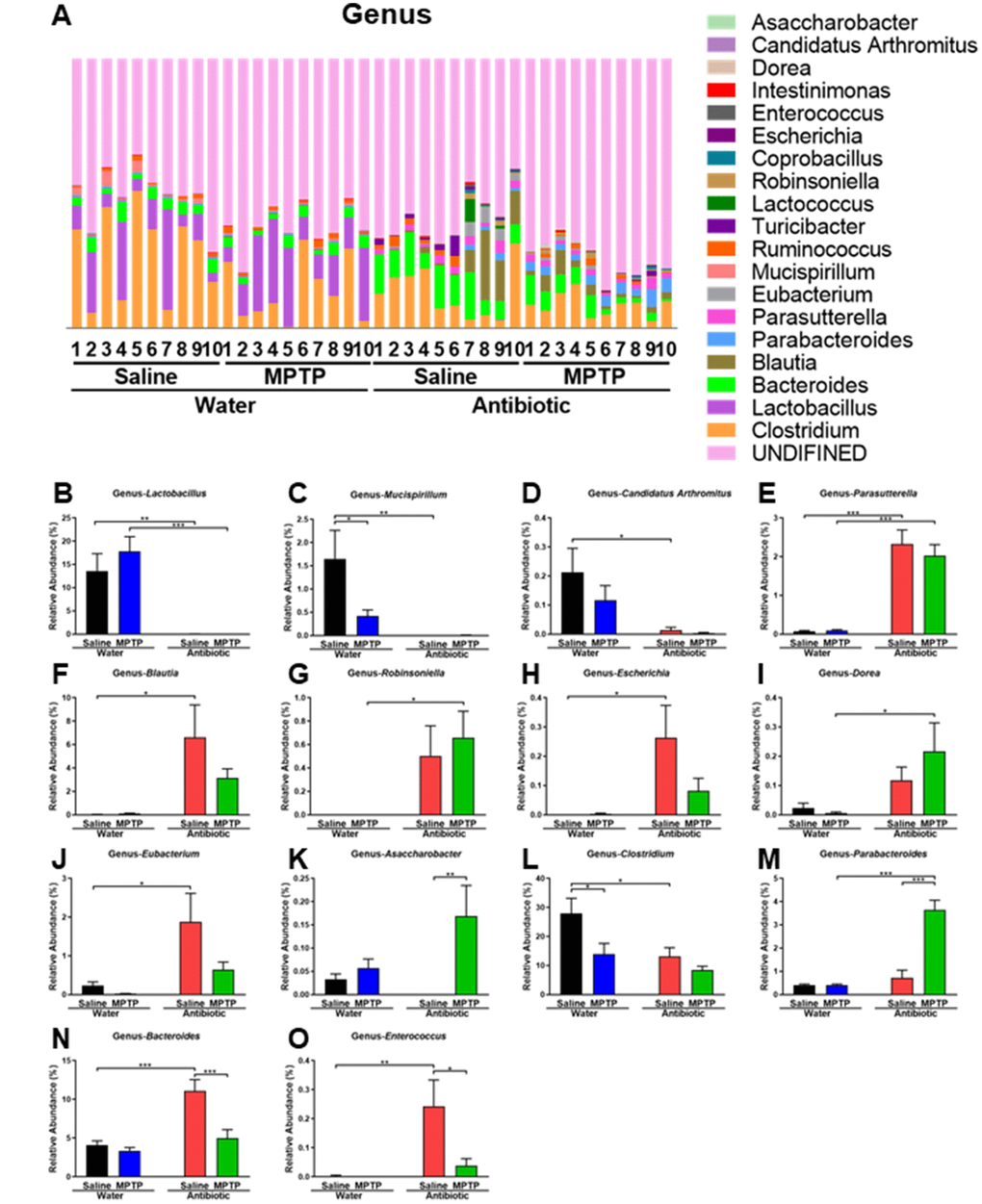
Figure 4. Altered gut bacteria composition at the genus level. (A) Relative abundance at the genus level. (B) Lactobacillus. (C) Mucispirillum. (D) Candidatus Arthromitus. (E) Parasutterella. (F) Blautia. (G) Robinsoniella. (H) Escherichia. (I) Dorea. (J) Eubacterium. (K) Asaccharobacter. (L) Clostridium. (M) Parabacteroides. (N) Bacteroides. (O) Enterococcus. Data are shown as mean ± S.E.M. (n = 10). **P < 0.01, ***P < 0.001. See the Supplementary Table 2 for detailed statistical analysis.
Gut microbiota composition at the species level in the four experimental groups is shown in Figure 5A. Lactobacillus murinus, Lactobacillus johnsonii, Mucispirillum schaedleri, and Candidatus Arthromitus sp. SFB-mouse decreased after antibiotic cocktail treatment (Figure 5B–5E). In contrast, Escherichia coli, Blautia sp. Ser8, and Robinsoniella peoriensis increased after antibiotic treatment (Figure 5F–5H). Clostridium sp. Clone-27, the most abundant species in control water + saline group mice, decreased in all other groups (Figure 5I). On the other hand, Blautia sp. canine oral taxon 143, Parabacteroides distasonis, Blautia coccoides, Clostridium sp. HGF2, and Clostridium bolteae increased in the antibiotic + MPTP group (Figure 5J–5N). In addition, Lactobacillus intestinalis and Lactobacillus reuteri, which increased in the water + MPTP group compared to the water + saline group, were markedly reduced after antibiotic treatment (Figure 5O and 5P). Interestingly, DAT immunoreactivity was negatively correlated with levels of Lactobacillus intestinalis (r = -0.38, P = 0.01) and Lactobacillus reuteri (r = -0.39, P = 0.01; Figure 5U and 5V). The antibiotic-induced increase in the abundance of Bacteroides acidifaciens, [Clostridium] cocleatum, and Enterococcus casseliflavus was largely reversed after MPTP administration (Figure 5Q–5S). In addition, MPTP restored Bacteroides sp. TP-5 to control levels after it had been reduced by antibiotic treatment (Figure 5T).
![Altered gut bacteria composition at the species level. (A) Relative abundance at the species level. (B) Lactobacillus murinus. (C) Lactobacillus johnsonii. (D) Mucispirillum schaedleri. (E) Candidatus Arthromitus sp. SFB-mouse. (F) Escherichia coli. (G) Blautia sp. Ser8. (H) Robinsoniella peoriensis. (I) Clostridium sp. Clone-27. (J) Blautia sp. canine oral taxon 143. (K) Parabacteroides distasonis. (L) Blautia coccoides. (M) Clostridium sp. HGF2. (N) Clostridium bolteae. (O) Lactobacillus intestinalis. (P) Lactobacillus reuteri. (Q) Bacteroides acidifaciens. (R) [Clostridium] cocleatum. (S) Enterococcus casseliflavus. (T) Bacteroides sp. TP-5. (U) Negative correlation (r = -0.38, P = 0.01) between Lactobacillus intestinalis and DAT immunoreactivity. (V) Negative correlation (r = - 0.39, P = 0.01) between Lactobacillus reuteri and DAT immunoreactivity. Data are shown as mean ± S.E.M. (n = 10). *P Supplementary Table 3 for detailed statistical analysis.](/article/102221/figure/f5/large)
Figure 5. Altered gut bacteria composition at the species level. (A) Relative abundance at the species level. (B) Lactobacillus murinus. (C) Lactobacillus johnsonii. (D) Mucispirillum schaedleri. (E) Candidatus Arthromitus sp. SFB-mouse. (F) Escherichia coli. (G) Blautia sp. Ser8. (H) Robinsoniella peoriensis. (I) Clostridium sp. Clone-27. (J) Blautia sp. canine oral taxon 143. (K) Parabacteroides distasonis. (L) Blautia coccoides. (M) Clostridium sp. HGF2. (N) Clostridium bolteae. (O) Lactobacillus intestinalis. (P) Lactobacillus reuteri. (Q) Bacteroides acidifaciens. (R) [Clostridium] cocleatum. (S) Enterococcus casseliflavus. (T) Bacteroides sp. TP-5. (U) Negative correlation (r = -0.38, P = 0.01) between Lactobacillus intestinalis and DAT immunoreactivity. (V) Negative correlation (r = - 0.39, P = 0.01) between Lactobacillus reuteri and DAT immunoreactivity. Data are shown as mean ± S.E.M. (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001. See the Supplementary Table 3 for detailed statistical analysis.