Sphingosine-1-phosphate promotes PDGF-dependent endothelial progenitor cell angiogenesis in human chondrosarcoma cells
Abstract
The malignant bone tumors that are categorized as chondrosarcomas display a high potential for metastasis in late-stage disease. Higher-grade chondrosarcomas contain higher levels of expression of platelet-derived growth factor (PDGF) and its receptor. The phosphorylation of sphingosine by sphingosine kinase enzymes SphK1 and SphK2 generates sphingosine-1-phosphate (S1P), which inhibits human chondrosarcoma cell migration, while SphK1 overexpression suppresses lung metastasis of chondrosarcoma. We sought to determine whether S1P mediates levels of PDGF-A expression and angiogenesis in chondrosarcoma. Surprisingly, our investigations found that treatment of chondrosarcoma cells with S1P and transfecting them with SphK1 cDNA increased PDGF-A expression and induced angiogenesis of endothelial progenitor cells (EPCs). Ras, Raf, MEK, ERK and AP-1 inhibitors and their small interfering RNAs (siRNAs) inhibited S1P-induced PDGF-A expression and EPC angiogenesis. Our results indicate that S1P promotes the expression of PDGF-A in chondrosarcoma via the Ras/Raf/MEK/ERK/AP-1 signaling cascade and stimulates EPC angiogenesis.
Introduction
The malignant bone tumors that constitute chondrosarcomas are not easy to diagnose or treat [1, 2], being characterized by poor responsiveness to conventional chemotherapy and radiotherapy [3], and high-grade chondrosarcoma has a poor prognosis with low survival rates despite wide surgical resection, which is considered to be the cornerstone of treatment [4].
Metastasis is the primary cause of cancer mortality [5–7]. Angiogenesis is vital for the development of cancer and metastasis [8–10]; the chief proangiogenic factor in these processes is vascular endothelial growth factor-A (VEGF-A) [11, 12]. Another crucial mediator of tumor angiogenesis and metastasis is the platelet-derived growth factor receptor (PDGFR); a positive correlation has been observed between levels of PDGFR-α expression and the aggressiveness of chondrosarcoma [13], so it is important to examine the molecular mechanisms underlying PDGF expression in human chondrosarcoma cells. Data are awaited from phase II trials investigating the efficacy of pazopanib, a potent PDGFR inhibitor, in different chondrosarcoma patient populations [14].
Sphingosine-1-phosphate (S1P), a platelet-derived lysophospholipid mediator, strongly inhibits PDGR-induced chemotaxis and cellular Rac activity in vascular smooth muscle cells [15]. Interestingly, deleting the S1P2 receptor promotes murine embryonic fibroblast migration towards S1P and also PDGF, which stimulates S1P production; S1P2 deletion also increases the enzymatic expression and activity of sphingosine kinase 1 (SphK1), which is responsible for producing S1P [16]. S1P/S1P receptor signaling has been discussed to regulate angiogenesis and vasculogenesis [17, 18]. S1P is known to regulate various cellular processes that are involved in cancer: SphK1 maintains tumor cell survival and promotes the progression of hormone-independent prostate and breast cancer [19, 20]; SphK1 overexpression stimulates Ras-dependent mechanisms that transform fibroblasts into fibrosarcoma; in estrogen receptor-positive breast cancer, high tumoral SphK1 expression is associated with poorer survival and shorter times to disease recurrence; and S1P promotes tumor neovascularization and induces inflammation involved in cancer progression [21]. Moreover, SphK1 and S1P encourage tumor growth and angiogenesis, metastasis and apoptotic resistance [22]. On the contrary, S1P also inhibits cancer cell migration by activation of S1P2- and ROCK-mediated vimentin S71 phosphorylation [23]. S1P is also capable of inhibiting endothelial cell angiogenesis [24], and of suppressing cell proliferation by inactivating Akt in keratinocytes [25] and prostate cancer cells [19]. We have previously demonstrated that S1P inhibits the migration of human chondrosarcoma cells and that SphK1 overexpression decreases metastasis to the lungs in a chondrosarcoma xenograft model [26]. Here, we sought to elucidate the relationship between S1P, PDGF-A expression and tumor angiogenesis, as well as characterize the molecular process whereby S1P induces PDGF-A-dependent angiogenesis in human chondrosarcoma cells.
Results
S1P enhances PDGF-A-dependent EPCs angiogenesis
The application of S1P to chondrosarcoma cell lines JJ012 and SW1353 concentration-dependently augmented PDGF-A mRNA and protein expression (Figure 1A, 1B). Evaluation of S1P-regulated angiogenesis in chondrosarcoma cells by EPC migration and tube formation assays [27] revealed that conditioned medium (CM) from S1P-treated chondrosarcoma cells stimulated EPC migration and tube formation (Figure 1C, 1D), whereas PDGF-A monoclonal antibody (mAb) treatment suppressed these events (Figure 1C, 1D). To confirm the role of S1P in EPC angiogenesis, the cells were transfected with SphK1 cDNA. We observed that overexpression of SphK1 cDNA increased levels of SphK1 protein expression (Figure 2B). Overexpression of SphK1 also increased PDGF-A mRNA and protein expression, EPC migration and tube formation (Figure 2). In addition, the PDGF-A mAb also blocked SphK1 facilitated EPC migration and tube formation (Figure 2C, 2D). Thus, S1P appears to stimulate EPC angiogenesis in a PDGF-A-dependent manner.
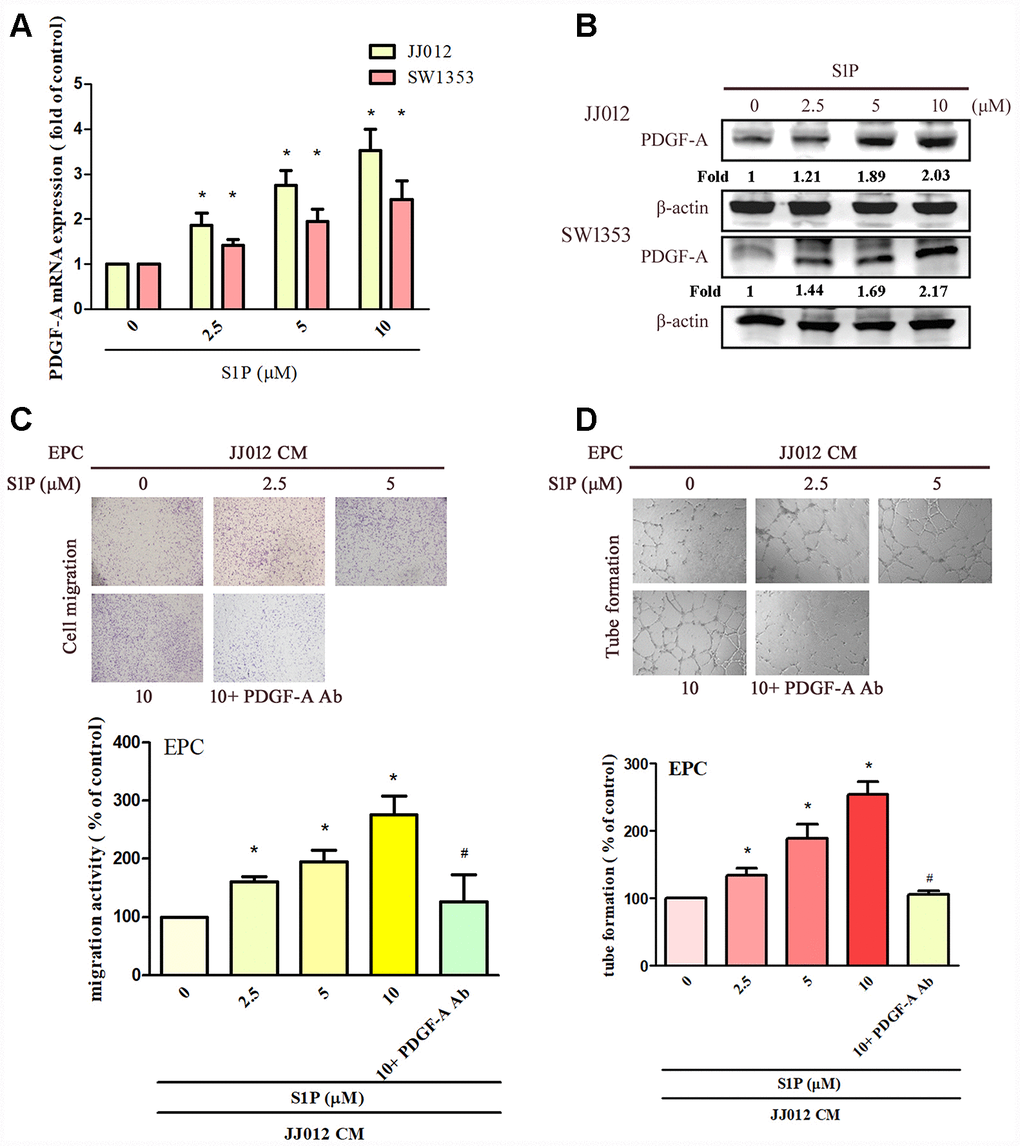
Figure 1. S1P increases PDGF-A expression and angiogenesis in human chondrosarcoma cells. (A, B) Chondrosarcoma cells were incubated with S1P (2.5–10 μM) for 24 h; PDGF-A expression was examined by qPCR and Western blot assays (n=4). (C, D) Chondrosarcoma cells were incubated with S1P for 24 h then stimulated with PDGF-A or IgG antibody (1 μg/ml) for 30 min. The conditioned medium (CM) was then collected and applied to endothelial progenitor cells (EPCs) (n=4). EPC migration and tube formation was measured. Results are expressed as the mean ± SEM. *p < 0.05 as compared with the control group; #p < 0.05 as compared with the S1P-treated group.
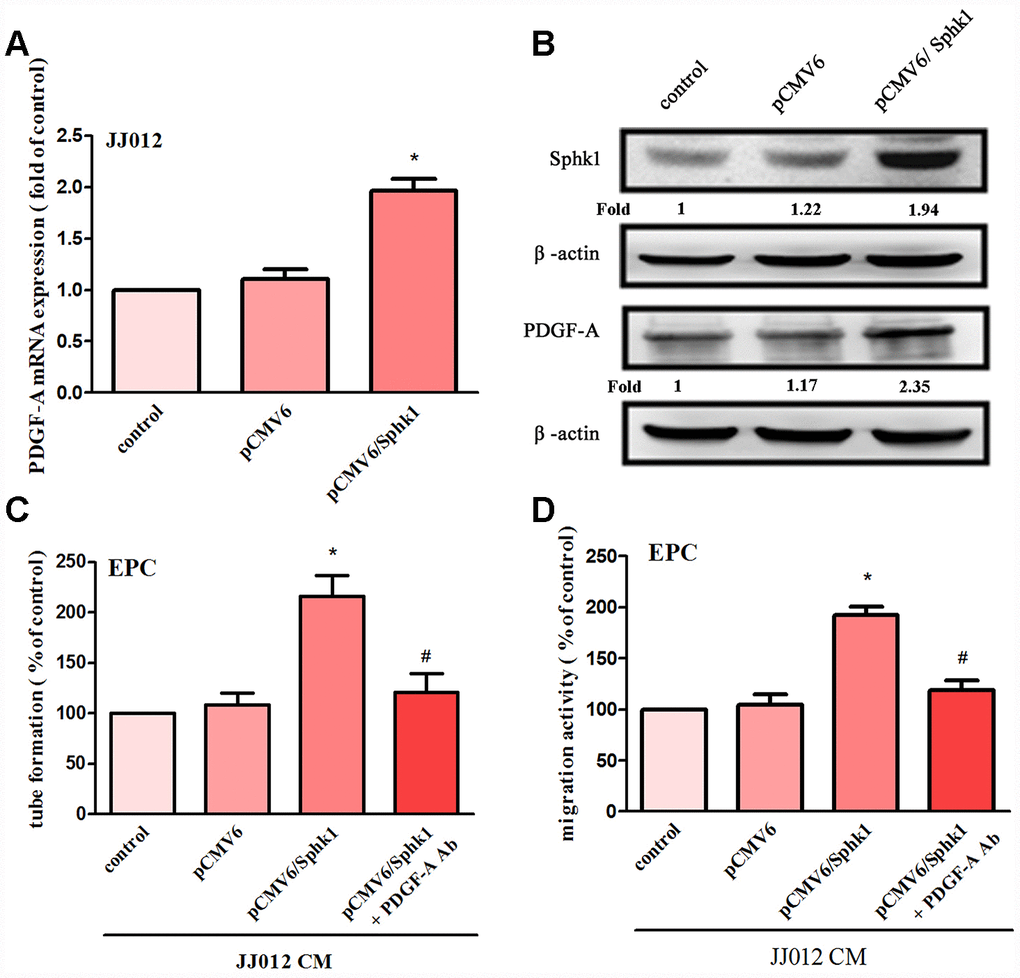
Figure 2. Overexpression of SphK1 facilitates in PDGF-A expression and angiogenesis in human chondrosarcoma. (A, B) Chondrosarcoma cells were transfected with SphK1 cDNA; SphK1 and PDGF-A expression was examined by qPCR and Western blot assays (n=5). (C, D) The CM was applied to EPCs and analyses assessed migratory and tube formation activity (n=4). Results are expressed as the mean ± SEM. *p < 0.05 as compared with the vector group.
S1P promotes PDGF-A-mediated angiogenesis through the Ras/Raf/MEK/ERK pathway
The Ras/Raf/MEK/ERK signaling pathway regulates tumor angiogenesis and metastasis [28, 29]. Treatment of cells with manumycin A (a Ras inhibitor) or GW5074 (a Raf inhibitor) suppressed S1P-enhanced PDGF-A expression, EPC migration and tube formation (Figure 3A–3C). Next, Ras and Raf siRNAs were used to confirm the results obtained from pharmacological inhibitors. We found that Ras and Raf siRNAs abolished S1P-mediated effects (Figure 3A–3C). Incubation of chondrosarcoma cells with S1P enhanced Ras kinase activity and Raf phosphorylation (Figure 3D). The Ras inhibitor also reduced S1P-enhanced phosphorylation of Raf (Figure 3E), indicating that Ras serves as an upstream molecule of Raf.
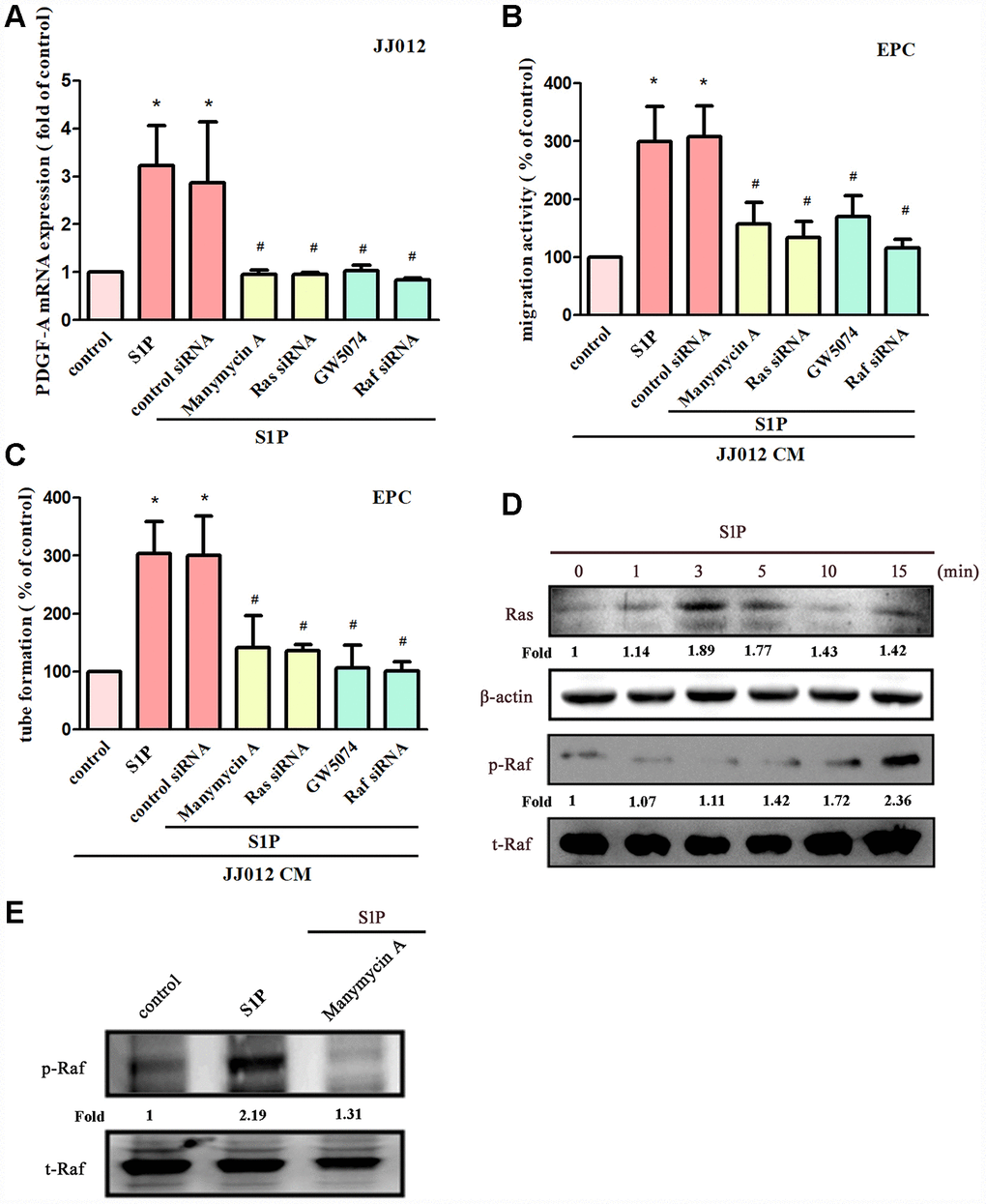
Figure 3. The Ras and Raf pathways mediate S1P-promoted PDGF-A expression and angiogenesis. (A) Cells were pretreated for 30 min with manumycin A (10 μM) and GW5074 (10 μM), or transfected with Ras and Raf siRNAs then stimulated with S1P (10 μM). PDGF-A expression was examined by qPCR assays (n=5). (B, C) The CM was applied to EPCs and analyses assessed migratory and tube formation activity (n=4). (D) JJ012 cells were incubated with S1P; Ras and Raf activity was examined by Western blot assay (n=3). (E) JJ012 cells were pretreated with manumycin A for 30 min, then stimulated with S1P and Raf phosphorylation was examined (n=3). Results are expressed as the mean ± SEM. *p < 0.05 as compared with the control group; #p < 0.05 as compared with the S1P-treated group.
MEK/ERK is a common downstream signaling pathway of Ras and Raf proteins [28, 30]. Incubating chondrosarcoma cells with MEK inhibitors (PD98059 and U0126) or siRNAs against MEK and ERK effectively reduced S1P-enhanced PDGF-A expression, EPC migration and tube formation (Figure 4A–4C). Stimulation of chondrosarcoma cells by S1P promoted MEK and ERK phosphorylation (Figure 4D). Conversely, S1P-induced phosphorylation of MEK and ERK was reduced when cells were pretreated with Ras, Raf and MEK inhibitors (Figure 4E, 4F). These results suggest that S1P acts via the Ras/Raf/MEK/ERK signaling mechanism to enhance levels of PDGF-A expression and angiogenic activity in human chondrosarcoma cells.
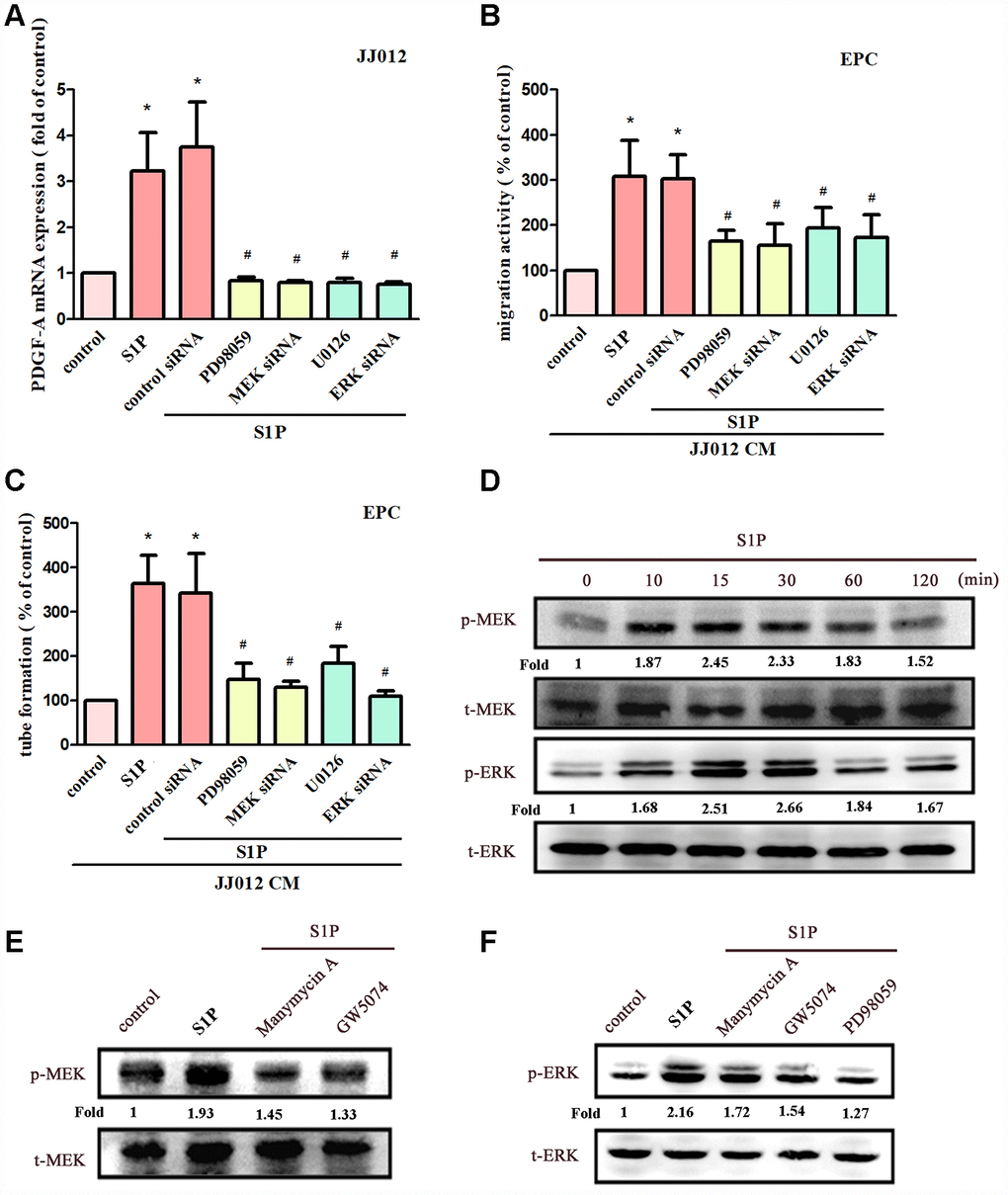
Figure 4. The MEK and ERK pathways mediated S1P-promoted PDGF-A expression and angiogenesis. (A) Cells were pretreated for 30 min with PD98059 (10 μM) and U0126 (5 μM), or transfected with MEK and ERK siRNAs, then stimulated with S1P (10 μM). PDGF-A expression was examined by qPCR assays (n=5). (B, C) The CM was applied to EPCs and analyses assessed migratory and tube formation activity (n=4). (D) JJ012 cells were incubated with S1P; MEK and ERK phosphorylation was examined by Western blot assay (n=3). (E, F) JJ012 cells were pretreated with manumycin A, GW5074 and PD98059 for 30 min, then stimulated with S1P (10 μM). MEK and ERK phosphorylation was examined (n=3). Results are expressed as the mean ± SEM. *p < 0.05 as compared with the control group; #p < 0.05 as compared with the S1P-treated group.
AP-1 transcriptional activity regulates S1P-promoted PDGF-A expression and angiogenesis
AP-1 appears to regulate PDGF gene expression [31]. We therefore examined whether AP-1 influences S1P-mediated PDGF-A expression in chondrosarcoma cells. Transfecting cells with an AP-1 inhibitor (tanshinone IIA) or c-Jun siRNA reduced S1P-promoted PDGF-A expression (Figure 5A); these compounds also suppressed S1P-induced EPC migration and tube formation (Figure 5B, 5C). S1P significantly promoted c-Jun phosphorylation (Figure 5D), which was reduced by pretreatment with Ras, Raf and MEK inhibitors (Figure 5E). To confirm that the Ras/Raf-1/MEK1/ERK signaling pathway mediated S1P-enhanced activation of AP-1, the AP-1 luciferase promoter plasmid was used. Treatment of cells with S1P augmented AP-1 luciferase activity, while pretreating the cells with Ras, Raf, MEK and ERK inhibitors or their siRNAs reduced S1P-induced AP-1 luciferase activity (Figure 5F, 5G). Activation of Ras, Raf-1, MEK1 and ERK appears to be involved in S1P-induced AP-1 activation in human chondrosarcoma cells.
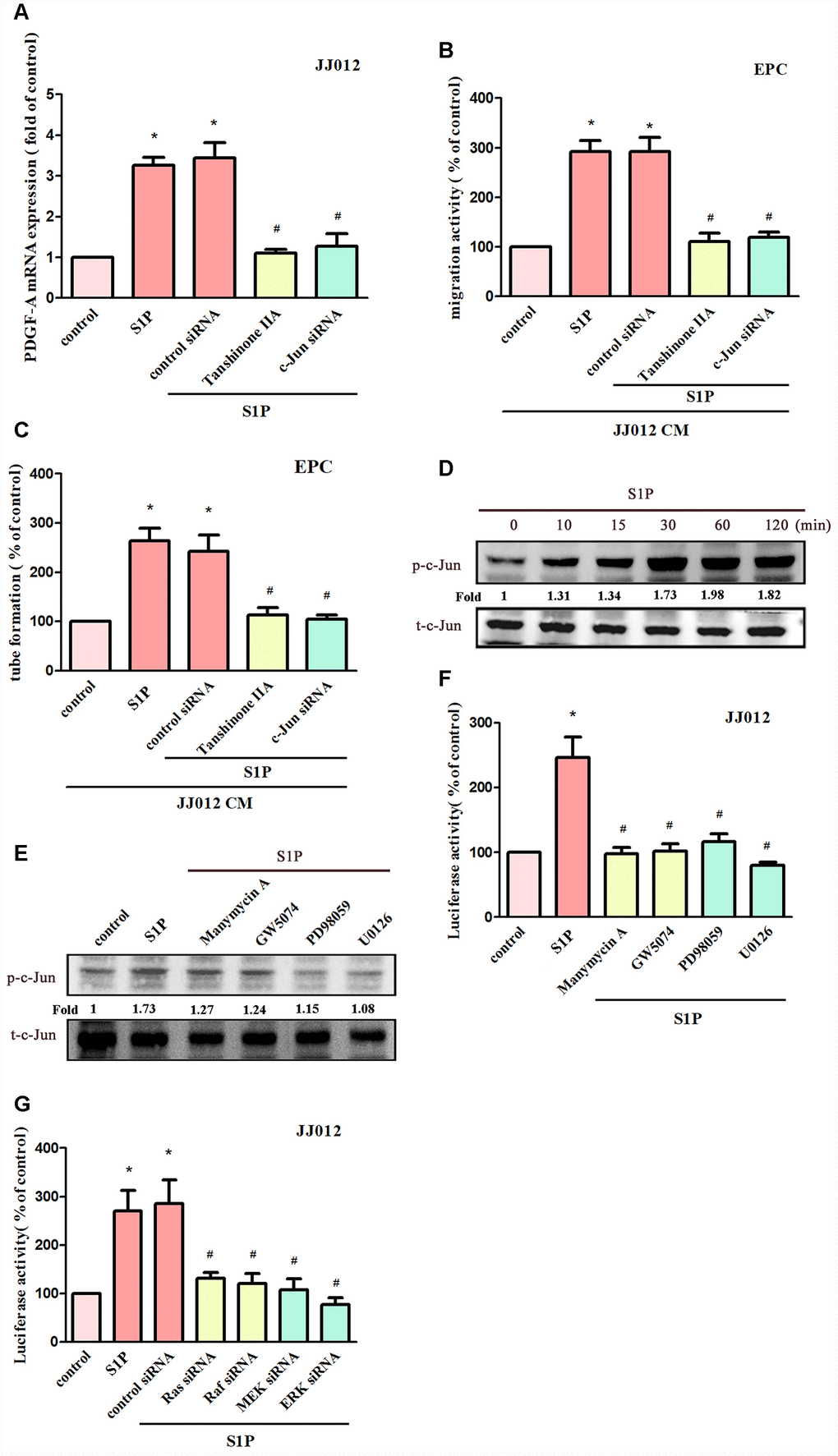
Figure 5. AP-1 is involved in S1P-facilitated PDGF-A expression and angiogenesis. (A) Cells were pretreated for 30 min with tanshinone IIA (3 μM), or transfected with c-Jun siRNA, then stimulated with S1P (10 μM). PDGF-A expression was examined by qPCR assays (n=5). (B, C) The CM was applied to EPCs and analyses assessed migratory and tube formation activity (n=4). (D) JJ012 cells were incubated with S1P (10 μM); c-Jun phosphorylation was examined by Western blot assay (n=3). (E) JJ012 cells were pretreated with manumycin A, GW5074, PD98059 and U0126 for 30 min, then stimulated with S1P (10 μM). The c-Jun phosphorylation was examined (n=3). (F, G) JJ012 cells were pretreated with Ras, Raf, MEK and ERK inhibitors or siRNAs, then stimulated with S1P (10 μM) and AP-1 Luciferase activity was examined (n=4). Results are expressed as the mean ± SEM. *p < 0.05 as compared with the control group; #p < 0.05 as compared with the S1P-treated group.
Discussion
Chondrosarcomas are heterogeneous, malignant bone neoplasms [26, 32] that are characterized by an increasing propensity for metastasis at higher pathological grades. Chemotherapy and radiotherapy are of limited effectiveness in chondrosarcoma; surgery is the favored therapeutic option [33]. Growth rates of many low- and moderate-grade chondrosarcomas are relatively slow; approximately 15% of all deaths due to metastasis occur more than 5 years after diagnosis [34]. This phenomenon offers a window of opportunity for the therapeutic prevention of chondrosarcoma metastasis. Our previous investigation reported that S1P inhibits migration, invasion and metastasis in human chondrosarcoma [26]. Here, we found that S1P enhanced PDGF-A expression in human chondrosarcoma and facilitated EPC angiogenesis through the Ras, Raf, MEK, ERK and AP-1 signaling pathways. The human umbilical vein endothelial cell (HUVEC) are common used model of tumor angiogenesis. However, few reports used this model to examine the angiogenic effect in chondrosarcoma. Whether EPCs or HUVEC are best model to study in the context of chondrosarcoma needs further study.
Human chondrosarcoma cell lines show upregulated PDGF and PDGFR activities, which are required for tumor growth and metastasis [35]. Pazopanib (a PDGFR inhibitor) was associated with clinical benefits in a patient with metastatic chondrosarcoma that had failed to respond to first-line chemotherapy [14]. Pazopanib has also demonstrated efficacy (prolonged disease stabilization for over 6 months) and good tolerability in 8 patients with progressive chondrosarcoma administered pazopanib 800 mg/day [36]. The PDGF/PDGFR axis is therefore a promising target for chondrosarcoma progression and metastasis. This paper reports that S1P enhanced PDGF-A mRNA and protein expression in both chondrosarcoma cell lines. Overexpression of SphK1 facilitated PDGF-A production. Otherwise, S1P also increases other angiogenic factors expression in chondrosarcoma, the PDGF-A is most upregulated (Supplementary Figure 1). Importantly, incubating EPCs with PDGF-A mAb antagonized S1P-induced migration and tube formation, indicating that S1P enhances EPC angiogenesis via a PDGF-A-dependent manner. Treatment of EPC with S1P slightly increased EPC tube formation (Supplementary Figure 2), indicating S1P also have direct effect in EPC angiogenesis.
S1P, a simple bioactive sphingolipid, is generated by SphK-induced phosphorylation of sphingosine. S1P regulates cancer-related processes such as autophagy, proliferation, angiogenesis and migration, by binding to its membranous receptors or by targeting the intracellular molecules [19, 22]. SphK1 upregulation has been identified in several cancers and is associated with poor survival prognosis [37]. We have previously demonstrated that S1P inhibits cellular migratory activity in human chondrosarcoma [26]. However, this investigation has demonstrated the opposite effect, in that S1P enhanced PDGF-A expression and EPC angiogenesis in human chondrosarcoma cell lines. We speculate that these apparently contradictory results may be explained in several ways. Firstly, angiogenesis is observed in the early stages of tumorigenesis, whereas endothelial cell migration generally occurs during tumor metastasis [38]. Secondly, EPC angiogenesis is only part of tumor angiogenesis; the endothelium cells may play a more important role than EPCs during tumor angiogenesis. Thirdly, the five S1P receptors may mediate different cell functions [16]. Finally, our investigation did not include an in vivo animal model to confirm the mediatory effects of S1P upon EPC angiogenesis.
The activation of the Ras/Raf/MEK/ERK signaling pathway is essential in many types of cancer for mediating multiple cellular functions, including cell survival, proliferation, migration and autophagy [29]. The Ras/Raf/MEK/ERK signaling pathway is also associated with tumor angiogenesis, as is seen with toluhydroquinone (2-methyl-1,4-hydroquinone), a marine-derived fungi, which reduces HUVEC angiogenesis via the Ras/Raf/MEK/ERK cascade [39]. In this study, we observed that S1P enhanced Ras, Raf, MEK and ERK activation, while Ras, Raf, MEK and ERK inhibitors and their siRNAs inhibit S1P-induced PDGF-A expression. It appears that the Ras/Raf/MEK/ERK signaling pathway is involved in S1P-mediated PDGF-A expression and angiogenesis. AP-1 has been indicated controls PDGF expression [31]. In current study, we also confirm AP-1 inhibitor or c-Jun siRNA abolished S1P-promoted PDGF-A expression. Otherwise, KLF5 has been previously shown to regulate PDGF-A expression through HIF1α [40]. Whether HIF1α also involve S1P-controled PDGF-A production are needs further examination.
The very poor prognosis for chondrosarcoma with metastatic disease makes it imperative that we can effectively prevent metastasis [41]. Our study shows that S1P enhances EPC angiogenesis in human chondrosarcoma, as a result of the upregulation of PDGF-A expression through the Ras/Raf/MEK/ERK/AP-1 signaling pathway (Figure 6). Clearly, therapeutic interventions are needed that focus on the functioning of the S1P signaling pathway and its relationship with PDGF-A in chondrosarcoma.
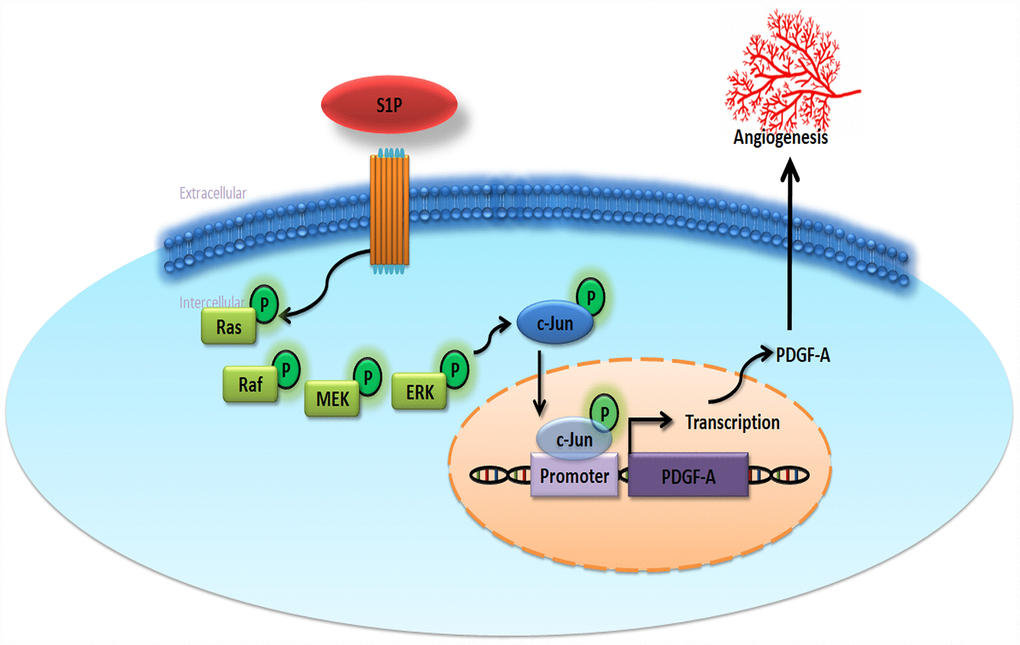
Figure 6. Schematic diagram summarizes the mechanisms of S1P-promoted tumor angiogenesis in chondrosarcoma. S1P facilitates PDGF-A production via the Ras/Raf/MEK/ERK/AP-1 signaling pathway in human chondrosarcoma cells and subsequently induces EPC angiogenesis.
Materials and Methods
Materials
S1P was obtained from Avanti Polar Lipid Inc. (Alabaster, AL, USA). We obtained SphK1 (GTX33516) antibody from Genetex (Irvine, CA, USA), Ras (SC-520), Raf (SC-133), MEK (SC-6250), ERK (SC-1647), PDGF-A (SC-9974), c-Jun (SC-74543), p-Raf (SC-101791), p-ERK (SC-7383) and p-c-Jun (SC-822) from Santa Cruz (Santa Cruz, CA, USA), and p-MEK (2338S) from Cell Signaling Technology (Danvers, MA, USA). ON-TARGETplus siRNAs were purchased from Dharmacon Research (Lafayette, CO, USA). SphK1 cDNA clone plasmid was purchased from OriGene (Rockville, MD, USA). Gibco-BRL Life Technologies (Grand Island, NY, USA) supplied fetal bovine serum (FBS), DMEM, α-MEM, and all other cell culture reagents. Promega (Madison, WI, USA) supplied the pSV-β-galactosidase vector and luciferase assay kits. The AP-1 luciferase plasmid was purchased from Stratagene (La Jolla, CA). All other chemicals or inhibitors were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The human chondrosarcoma cell line JJ012 was kindly supplied by Dr. Sean P. Scully’s laboratory at the University of Miami School of Medicine (Miami, FL, USA). The human chondrosarcoma cell line SW1353 was purchased from the American Type Culture Collection (Manassas, VA, USA). Chondrosarcoma cell culture conditions were recorded as previously described [42]. Human EPCs were isolated and cultured by a standard method as previously described [43, 44]. This study was approved by the Institutional Review Board of Mackay Medical College, New Taipei City, Taiwan (P1000002).
Preparation of conditioned medium (CM)
Human chondrosarcoma cells were plated in 6-well plates at a density of 2×105 cells/well in culture medium, grown to 80% confluence. Human chondrosarcoma cells were pretreated with pharmacological inhibitors, or transfected with siRNA followed by stimulation with S1P for 24 h. After treatment, cells were washed and changed to serum-free medium. CM was then collected 2 days after the change of medium and stored at −80°C until use.
EPC tube formation assay
The tube formation assay was performed using Matrigel-coated (BD Biosciences, Bedford, MA, USA) 48-well plates. EPCs were resuspended at a density of 2 × 104/200 μL in culture medium (50% EGM-MV2 medium and 50% chondrosarcoma cell CM) and added to the wells. Measurement of tube formation examined the differentiation and formation of capillary-like tubules on EPCs, according to previously described procedures [45, 46].
EPC migration assay
Transwell inserts (8-μm pore size; Costar, NY, USA) were used for migration determination. Approximately 5 × 103 cells were added to the upper chamber in 200 μL of 10% FBS MV2 complete medium. The lower chamber contained 150 μL 20% FBS MV2 complete medium and 150 μl CM. EPC migratory ability was assayed using the method from our previous works [45, 47].
Western blot analysis
Chondrosarcoma cells were seeded on 6-well plates at a density of 3×105 cells/well, grown to 80% confluence and then handle different conditions according to experimental needs the next day. Cell lysates underwent electrophoresis with SDS-PAGE and were transferred to PVDF membranes according to the method described in our previous studies [48, 49]. After blocking the blots with 4% bovine serum albumin, the blots were treated with primary antibody and then peroxidase-conjugated secondary antibody. Visualizations of the blots were accomplished by enhanced chemiluminescence using the UVP Biospectrum system (UVP, Upland, CA, USA).
Quantitative real-time PCR (qPCR) of mRNA
Human chondrosarcoma cells were plated in 6-well plates at a density of 2×105 cells/well in culture medium, grown to 80% confluence and then handle different conditions according to experimental needs the next day. Total RNA was extracted from chondrosarcoma cells using TRIzol reagent. qPCR analysis was conducted according to an established protocol [50, 51].
Ras kinase activity
Chondrosarcoma cells were seeded on 6-well plates at a density of 3×105 cells/well, grown to 80% confluence. Cells were treated with S1P then the activation of Ras (Ras-GTP) was detected using the Ras-binding domain of Raf-1 to pull down active Ras (Ras Activation Assay Kit; Millipore, CA, USA), according to the manufacturer's recommendations. Following separation by SDS-PAGE, proteins were transferred to membranes that were probed with an anti-RAS antibody [34].
siRNA transient transfection and luciferase reporter assay
Chondrosarcoma cells were seeded on 12-well plates at a density of 1×105 cells/well, grown to 80% confluence and transfected the next day. Cells were co-transfected with 0.8 μg AP-1-luciferase reporter gene construct and 0.4 μg β-galactosidase using Lipofectamine 2000, as per the manufacturer's instructions. After 24 h of transfection, the cells were exposed to S1P. Luciferase activity was determined using the luciferase assay kit [52, 53].
Statistical analysis
All data are presented as means ± standard errors of the means (SEMs) of at least three independent experiments. The Student’s t-test determined statistical differences between samples and the Bonferroni post hoc procedure was performed for a one-way analysis of variance (ANOVA) of statistical comparisons between more than two samples, and p-values of less than 0.05 were considered significant.
Conflicts of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflicts with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Funding
This work was supported by a grant from China Medical University (CMU 108-S-08).
References
-
1.
Group ES, and ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014 (Suppl 3); 25:iii113–23. https://doi.org/10.1093/annonc/mdu256 [PubMed]
-
2.
Su CM, Tang CH, Chi MJ, Lin CY, Fong YC, Liu YC, Chen WC, Wang SW. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochem Pharmacol. 2018; 154:234–42. https://doi.org/10.1016/j.bcp.2018.05.001 [PubMed]
-
3.
Jamil N, Howie S, Salter DM. Therapeutic molecular targets in human chondrosarcoma. Int J Exp Pathol. 2010; 91:387–93. https://doi.org/10.1111/j.1365-2613.2010.00749.x [PubMed]
-
4.
MacDonald IJ, Lin CY, Kuo SJ, Su CM, Tang CH. An update on current and future treatment options for chondrosarcoma. Expert Rev Anticancer Ther. 2019; 19:773–86. https://doi.org/10.1080/14737140.2019.1659731 [PubMed]
-
5.
Welch DR, Hurst DR. Defining the Hallmarks of Metastasis. Cancer Res. 2019; 79:3011–27. https://doi.org/10.1158/0008-5472.CAN-19-0458 [PubMed]
-
6.
Wang M, Chao CC, Chen PC, Liu PI, Yang YC, Su CM, Huang WC, Tang CH. Thrombospondin enhances RANKL-dependent osteoclastogenesis and facilitates lung cancer bone metastasis. Biochem Pharmacol. 2019; 166:23–32. https://doi.org/10.1016/j.bcp.2019.05.005 [PubMed]
-
7.
Tai HC, Lee TH, Tang CH, Chen LP, Chen WC, Lee MS, Chen PC, Lin CY, Chi CW, Chen YJ, Lai CT, Chen SS, Liao KW, et al. Phomaketide A Inhibits Lymphangiogenesis in Human Lymphatic Endothelial Cells. Mar Drugs. 2019; 17:17. https://doi.org/10.3390/md17040215 [PubMed]
-
8.
Giner F, López-Guerrero JA, Machado I, García-Casado Z, Peydró-Olaya A, Llombart-Bosch A. The early stages of tumor angiogenesis in human osteosarcoma: a nude mice xenotransplant model. Virchows Arch. 2015; 467:193–201. https://doi.org/10.1007/s00428-015-1791-y [PubMed]
-
9.
Chen JC, Fong YC, Tang CH. Novel strategies for the treatment of chondrosarcomas: targeting integrins. Biomed Res Int. 2013; 2013:396839. https://doi.org/10.1155/2013/396839 [PubMed]
-
10.
Chang LC, Yu YL. Dietary components as epigenetic-regulating agents against cancer. Biomedicine (Taipei). 2016; 6:2. https://doi.org/10.7603/s40681-016-0002-8 [PubMed]
-
11.
Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011; 2:1117–33. https://doi.org/10.1177/1947601911423654 [PubMed]
-
12.
Padma VV. An overview of targeted cancer therapy. Biomedicine (Taipei). 2015; 5:19. https://doi.org/10.7603/s40681-015-0019-4 [PubMed]
-
13.
Sulzbacher I, Birner P, Trieb K, Mühlbauer M, Lang S, Chott A. Platelet-derived growth factor-alpha receptor expression supports the growth of conventional chondrosarcoma and is associated with adverse outcome. Am J Surg Pathol. 2001; 25:1520–27. https://doi.org/10.1097/00000478-200112000-00008 [PubMed]
-
14.
Tsavaris O, Economopoulou P, Kotsantis I, Reppas L, Avgerinou C, Spathas N, Prevezanou M, Psyrri A. Clinical Benefit of Pazopanib in a Patient with Metastatic Chondrosarcoma: A Case Report and Review of the Literature. Front Oncol. 2018; 8:45. https://doi.org/10.3389/fonc.2018.00045 [PubMed]
-
15.
Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002; 90:325–32. https://doi.org/10.1161/hh0302.104455 [PubMed]
-
16.
Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005; 25:4237–49. https://doi.org/10.1128/MCB.25.10.4237-4249.2005 [PubMed]
-
17.
Argraves KM, Wilkerson BA, Argraves WS. Sphingosine-1-phosphate signaling in vasculogenesis and angiogenesis. World J Biol Chem. 2010; 1:291–97. https://doi.org/10.4331/wjbc.v1.i10.291 [PubMed]
-
18.
Takuwa Y, Du W, Qi X, Okamoto Y, Takuwa N, Yoshioka K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J Biol Chem. 2010; 1:298–306. https://doi.org/10.4331/wjbc.v1.i10.298 [PubMed]
-
19.
Huang YL, Chang CL, Tang CH, Lin YC, Ju TK, Huang WP, Lee H. Extrinsic sphingosine 1-phosphate activates S1P5 and induces autophagy through generating endoplasmic reticulum stress in human prostate cancer PC-3 cells. Cell Signal. 2014; 26:611–18. https://doi.org/10.1016/j.cellsig.2013.11.024 [PubMed]
-
20.
Wang W, Hind T, Lam BW, Herr DR. Sphingosine 1-phosphate signaling induces SNAI2 expression to promote cell invasion in breast cancer cells. FASEB J. 2019; 33:7180–91. https://doi.org/10.1096/fj.201801635R [PubMed]
-
21.
Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, Jiang HR, Ubhi S, Pyne S. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv Biol Regul. 2016; 60:151–59. https://doi.org/10.1016/j.jbior.2015.09.001 [PubMed]
-
22.
Huang YL, Lin HS, Chen SU, Lee H. Tyrosine sulphation of sphingosine 1-phosphate 1 (S1P1) is required for S1P-mediated cell migration in primary cultures of human umbilical vein endothelial cells. J Biochem. 2009; 146:815–20. https://doi.org/10.1093/jb/mvp131 [PubMed]
-
23.
Hyder CL, Kemppainen K, Isoniemi KO, Imanishi SY, Goto H, Inagaki M, Fazeli E, Eriksson JE, Törnquist K. Sphingolipids inhibit vimentin-dependent cell migration. J Cell Sci. 2015; 128:2057–69. https://doi.org/10.1242/jcs.160341 [PubMed]
-
24.
Mascall KS, Small GR, Gibson G, Nixon GF. Sphingosine-1-phosphate-induced release of TIMP-2 from vascular smooth muscle cells inhibits angiogenesis. J Cell Sci. 2012; 125:2267–75. https://doi.org/10.1242/jcs.099044 [PubMed]
-
25.
Kim DS, Kim SY, Kleuser B, Schäfer-Korting M, Kim KH, Park KC. Sphingosine-1-phosphate inhibits human keratinocyte proliferation via Akt/protein kinase B inactivation. Cell Signal. 2004; 16:89–95. https://doi.org/10.1016/S0898-6568(03)00114-1 [PubMed]
-
26.
Tsai CH, Yang DY, Lin CY, Chen TM, Tang CH, Huang YL. Sphingosine-1-phosphate suppresses chondrosarcoma metastasis by upregulation of tissue inhibitor of metalloproteinase 3 through suppressing miR-101 expression. Mol Oncol. 2017; 11:1380–98. https://doi.org/10.1002/1878-0261.12106 [PubMed]
-
27.
Su CM, Hsu CJ, Tsai CH, Huang CY, Wang SW, Tang CH. Resistin Promotes Angiogenesis in Endothelial Progenitor Cells Through Inhibition of MicroRNA206: Potential Implications for Rheumatoid Arthritis. Stem Cells. 2015; 33:2243–55. https://doi.org/10.1002/stem.2024 [PubMed]
-
28.
Tang CH, Tsai CC. CCL2 increases MMP-9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol. 2012; 83:335–44. https://doi.org/10.1016/j.bcp.2011.11.013 [PubMed]
-
29.
Yang S, Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett. 2017; 13:1041–47. https://doi.org/10.3892/ol.2017.5557 [PubMed]
-
30.
Tang CH, Chen CF, Chen WM, Fong YC. IL-6 increases MMP-13 expression and motility in human chondrosarcoma cells. J Biol Chem. 2011; 286:11056–66. https://doi.org/10.1074/jbc.M110.204081 [PubMed]
-
31.
Wygrecka M, Zakrzewicz D, Taborski B, Didiasova M, Kwapiszewska G, Preissner KT, Markart P. TGF-β1 induces tissue factor expression in human lung fibroblasts in a PI3K/JNK/Akt-dependent and AP-1-dependent manner. Am J Respir Cell Mol Biol. 2012; 47:614–27. https://doi.org/10.1165/rcmb.2012-0097OC [PubMed]
-
32.
Wu MH, Huang PH, Hsieh M, Tsai CH, Chen HT, Tang CH. Endothelin-1 promotes epithelial-mesenchymal transition in human chondrosarcoma cells by repressing miR-300. Oncotarget. 2016; 7:70232–46. https://doi.org/10.18632/oncotarget.11835 [PubMed]
-
33.
Shemesh SS, Acevedo-Nieves JD, Pretell-Mazzini J. Treatment strategies for central low-grade chondrosarcoma of long bones: a systematic review of the literature and meta-analysis. Musculoskelet Surg. 2018; 102:95–109. https://doi.org/10.1007/s12306-017-0507-7 [PubMed]
-
34.
Chen JC, Chen YJ, Lin CY, Fong YC, Hsu CJ, Tsai CH, Su JL, Tang CH. Amphiregulin enhances alpha6beta1 integrin expression and cell motility in human chondrosarcoma cells through Ras/Raf/MEK/ERK/AP-1 pathway. Oncotarget. 2015; 6:11434–46. https://doi.org/10.18632/oncotarget.3397 [PubMed]
-
35.
Boehme KA, Schleicher SB, Traub F, Rolauffs B. Chondrosarcoma: A Rare Misfortune in Aging Human Cartilage? The Role of Stem and Progenitor Cells in Proliferation, Malignant Degeneration and Therapeutic Resistance. Int J Mol Sci. 2018; 19:19. https://doi.org/10.3390/ijms19010311 [PubMed]
-
36.
Jones RL, Katz D, Loggers ET, Davidson D, Rodler ET, Pollack SM. Clinical benefit of antiangiogenic therapy in advanced and metastatic chondrosarcoma. Med Oncol. 2017; 34:167. https://doi.org/10.1007/s12032-017-1030-2 [PubMed]
-
37.
Xu Y, Dong B, Huang J, Kong W, Xue W, Zhu Y, Zhang J, Huang Y. Sphingosine kinase 1 is overexpressed and promotes adrenocortical carcinoma progression. Oncotarget. 2016; 7:3233–44. https://doi.org/10.18632/oncotarget.6564 [PubMed]
-
38.
Bielenberg DR, Zetter BR. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015; 21:267–73. https://doi.org/10.1097/PPO.0000000000000138 [PubMed]
-
39.
Kim NH, Jung HI, Choi WS, Son BW, Seo YB, Choi JS, Kim GD. Toluhydroquinone, the secondary metabolite of marine algae symbiotic microorganism, inhibits angiogenesis in HUVECs. Biomed Pharmacother. 2015; 70:129–39. https://doi.org/10.1016/j.biopha.2015.01.004 [PubMed]
-
40.
Ci X, Xing C, Zhang B, Zhang Z, Ni JJ, Zhou W, Dong JT. KLF5 inhibits angiogenesis in PTEN-deficient prostate cancer by attenuating AKT activation and subsequent HIF1α accumulation. Mol Cancer. 2015; 14:91. https://doi.org/10.1186/s12943-015-0365-6 [PubMed]
-
41.
Tsai CH, Tsai HC, Huang HN, Hung CH, Hsu CJ, Fong YC, Hsu HC, Huang YL, Tang CH. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget. 2015; 6:258–70. https://doi.org/10.18632/oncotarget.2724 [PubMed]
-
42.
Lin CY, Wang SW, Chen YL, Chou WY, Lin TY, Chen WC, Yang CY, Liu SC, Hsieh CC, Fong YC, Wang PC, Tang CH. Brain-derived neurotrophic factor promotes VEGF-C-dependent lymphangiogenesis by suppressing miR-624-3p in human chondrosarcoma cells. Cell Death Dis. 2017; 8:e2964. https://doi.org/10.1038/cddis.2017.354 [PubMed]
-
43.
Chung CH, Chang CH, Chen SS, Wang HH, Yen JY, Hsiao CJ, Wu NL, Chen YL, Huang TF, Wang PC, Yeh HI, Wang SW. Butein Inhibits Angiogenesis of Human Endothelial Progenitor Cells via the Translation Dependent Signaling Pathway. Evid Based Complement Alternat Med. 2013; 2013:943187. https://doi.org/10.1155/2013/943187 [PubMed]
-
44.
Wu MH, Huang CY, Lin JA, Wang SW, Peng CY, Cheng HC, Tang CH. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene. 2014; 33:1725–35. https://doi.org/10.1038/onc.2013.109 [PubMed]
-
45.
Lee HP, Chen PC, Wang SW, Fong YC, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, Li TM, Tang CH. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J Funct Foods. 2019; 52:537–44. https://doi.org/10.1016/j.jff.2018.11.040
-
46.
Lee HP, Wang SW, Wu YC, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, Yin MC, Li TM, Tang CH. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric Immunol. 2019; 30:1033–45. https://doi.org/10.1080/09540105.2019.1660623
-
47.
Liu SC, Chuang SM, Hsu CJ, Tsai CH, Wang SW, Tang CH. CTGF increases vascular endothelial growth factor-dependent angiogenesis in human synovial fibroblasts by increasing miR-210 expression. Cell Death Dis. 2014; 5:e1485. https://doi.org/10.1038/cddis.2014.453 [PubMed]
-
48.
Tang CH, Hsu CJ, Fong YC. The CCL5/CCR5 axis promotes interleukin-6 production in human synovial fibroblasts. Arthritis Rheum. 2010; 62:3615–24. https://doi.org/10.1002/art.27755 [PubMed]
-
49.
Wang SW, Liu SC, Sun HL, Huang TY, Chan CH, Yang CY, Yeh HI, Huang YL, Chou WY, Lin YM, Tang CH. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis. 2015; 36:104–14. https://doi.org/10.1093/carcin/bgu218 [PubMed]
-
50.
Huang CC, Chiou CH, Liu SC, Hu SL, Su CM, Tsai CH, Tang CH. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: implications for the treatment of rheumatoid arthritis. J Pineal Res. 2019; 66:e12560. https://doi.org/10.1111/jpi.12560 [PubMed]
-
51.
Liu SC, Tsai CH, Wu TY, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, Yin MC, Wu YC, Tang CH. Soya-cerebroside reduces IL-1 beta-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: implications for the treatment of osteoarthritis. Food Agric Immunol. 2019; 30:620–32. https://doi.org/10.1080/09540105.2019.1611745
-
52.
Yang YC, Chiou PC, Chen PC, Liu PY, Huang WC, Chao CC, Tang CH. Melatonin reduces lung cancer stemness through inhibiting of PLC, ERK, p38, β-catenin, and Twist pathways. Environ Toxicol. 2019; 34:203–09. https://doi.org/10.1002/tox.22674 [PubMed]
-
53.
Wu TJ, Lin CY, Tsai CH, Huang YL, Tang CH. Glucose suppresses IL-1β-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ Toxicol. 2018; 33:1061–68. https://doi.org/10.1002/tox.22618 [PubMed]