In vitro
To investigate if the probiotic formulation SLAB51 contained neuroprotective components, an in vitro model of PD was developed, and neuroprotective and neuronal death pathways were analyzed. The induction of the dopaminergic phenotype of SH-SY5Y neuroblastoma cells, described in the Methods section, was analyzed by contrast phase microscopy, Tyrosine Hydroxylase (TH) expression, dopamine production and immunofluorescence (Figure 1). It is possible to observe that, upon retinoic acid (RA)/phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment, cells displayed neuronal morphology, expressed higher levels of TH, produced dopamine and expressed neuronal markers, such as β-tubulin III and growth associated protein-43 (GAP-43). 6-hydroxydopamine (6-OHDA) concentration able to induce a significant decrease of cell viability was evaluated by MTS assay (not shown). On this basis, 35 μM 6-OHDA was chosen for the subsequent experiments, since a 50% mortality with this dosage was obtained.
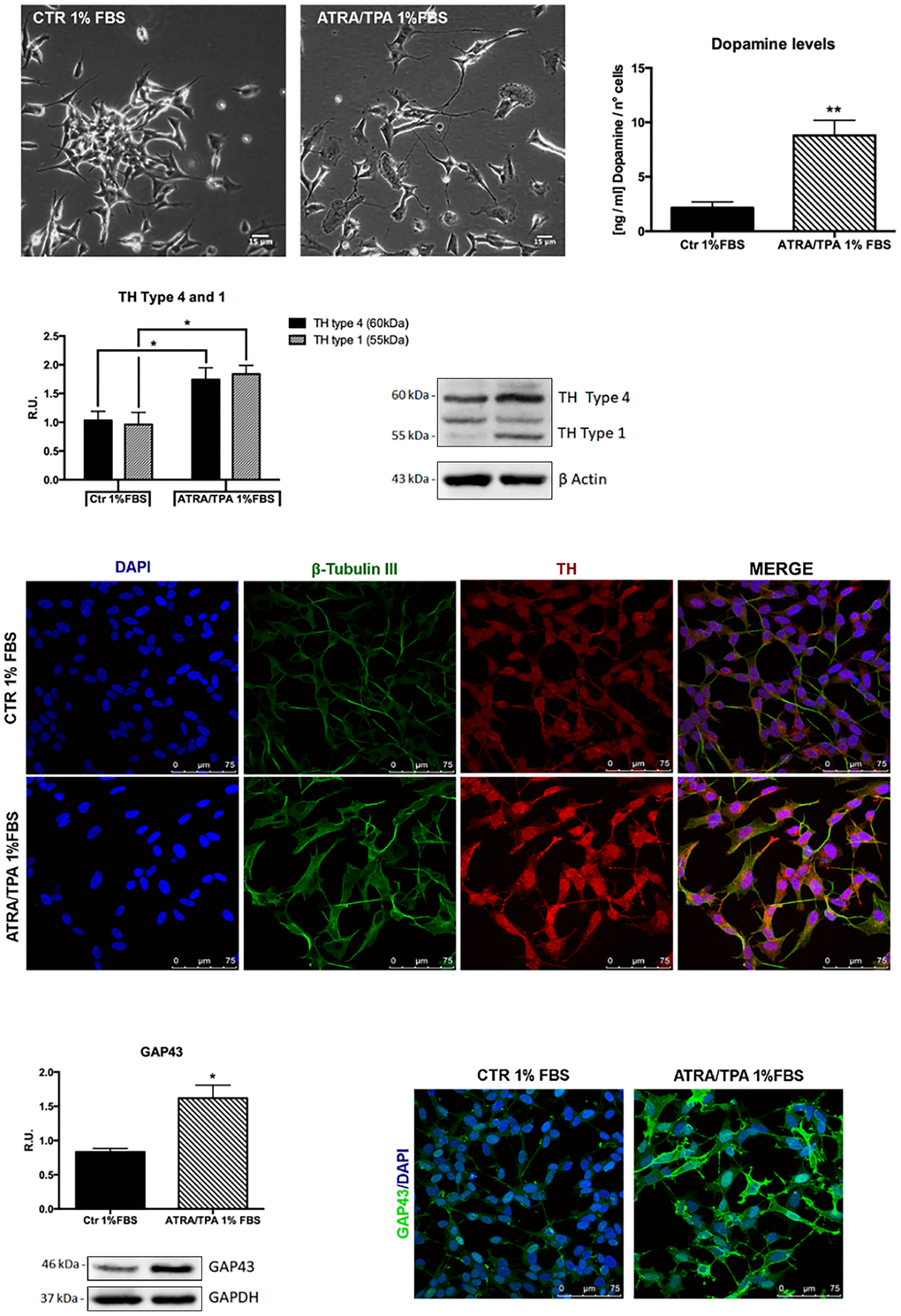
Figure 1. Dopaminergic phenotype of SH-SY5Y neuroblastoma cells. Contrast phase microscopy of differentiated with ATRA/TPA and not differentiated SH-SY5Y cells and histogram showing dopamine production. Western blotting for TH. Immunofluorescence of β-tubulin III and TH. Western blotting and immunofluorescence for GAP43. Results are mean ± SE of 3 different experiments (n=3). *p< 0,05, **p< 0,005 vs. ATRA/TPA.
SLAB51 lysates do not shown toxic effect, as demonstrated by cell viability test on differentiated SH-SY5Y; thus, basing on these preliminary results, 0.1 mg/ml of extract was set as testing concentration for the subsequent experiments. In Figure 2, the MTS assay of cells treated with 35 μM 6-OHDA and SLAB51 is shown. As evident, 6-OHDA led to 50% mortality, while SLAB51 was able to counteract 6-OHDA-induced injury and to restore control conditions.
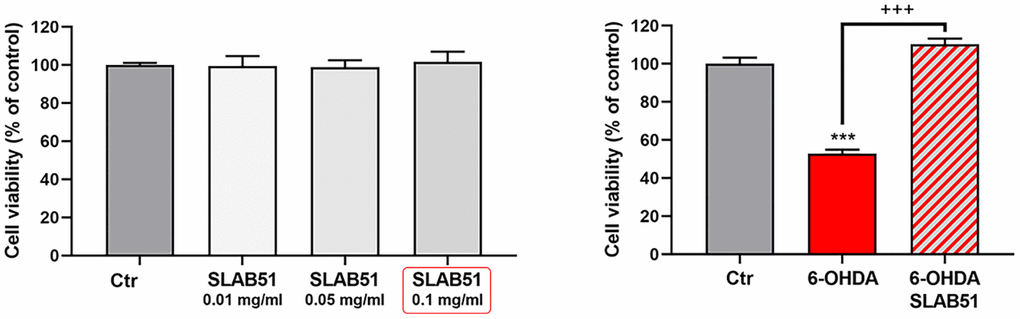
Figure 2. MTS assay of cells treated with different concentration of SLAB51 (left). MTS assay of cells treated with 35 μM 6-OHDA and 35 μM 6-OHDA and SLAB51 0.1mg/ml (right). Data are mean ± SE of three different experiments run in quadruplicate (n=3). *** p< 0.0005 vs Ctr; +++ p< 0.0005 vs 6-OHDA.
Since it is worth noting that BDNF pathway is involved in neuroprotection and neuronal survival, we first analyzed, by Western blotting, the protein levels of mature BDNF (mBDNF), phosphorylated tyrosine receptor kinase B (p-TrkB), phosphorylated cAMP response element-binding protein (p-CREB) and phosphorylated extracellular-signal-regulated kinase 5 (p-ERK5). In the presence of 6-OHDA, mBDNF and its specific receptor TrkB as well the survival kinase ERK5 were significantly decreased with respect to control neurons, while the presence of SLAB51 restored the control levels (Figure 3), suggesting a protective action exerted by the probiotic. Further, p-CREB, which is known to control mBDNF levels and the survival pathway phosphoinositide 3-kinase (PI3K)/ phosphorylated protein kinase B (p-Akt), was dramatically decreased upon 6-OHDA and restored at control levels by SLAB51 (Figure 3).
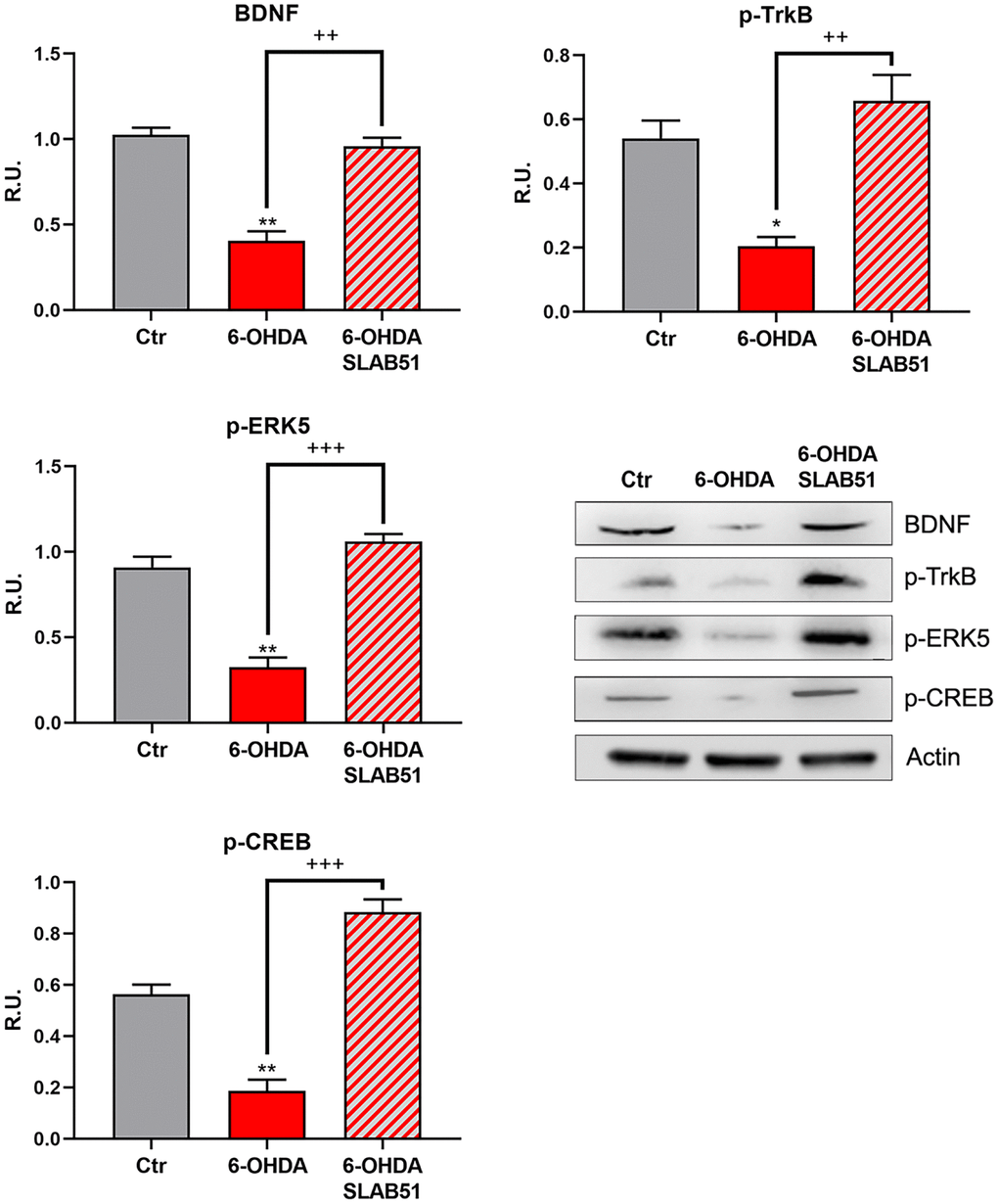
Figure 3. WB and relative densitometric analysis for Ctr, 6-OHDA and 6-OHDA+SLAB51 for mBDNF, p-TrkB, p-ERK5, p-CREB. Results are mean ± SE of 3 different experiments (n=3). *p< 0.05; ** p< 0.005 vs Ctr; ++ p< 0.005, +++ p< 0.0005 vs 6-OHDA. Representative WB figures are shown.
Finally, PI3K/Akt pathway, which is involved in neuronal survival and CREB phosphorylation, as well as the postsynaptic density protein 95 (PSD95) appeared strongly downregulated by 6-OHDA, while the presence of SLAB51 counteracted this effect (Figure 4), thus suggesting that the lysate is able to ameliorate the neuronal synaptic plasticity as well the neuronal survival.
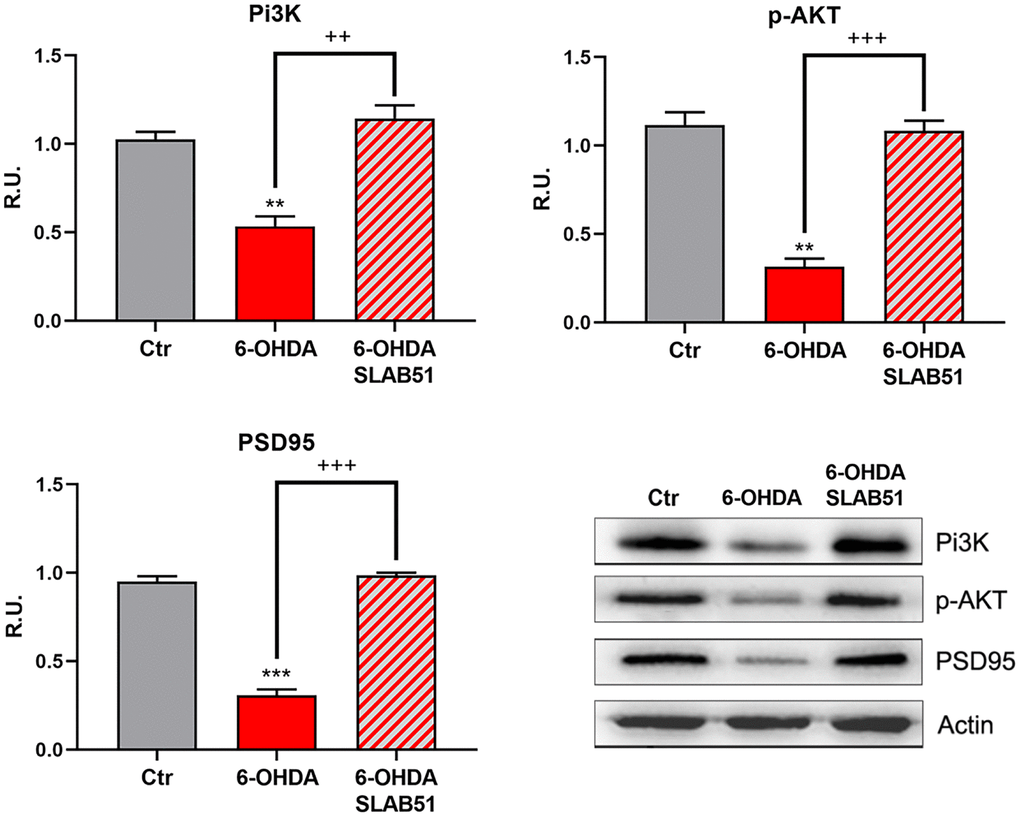
Figure 4. WB and relative densitometric analysis for Ctr, 6-OHDA and 6-OHDA+SLAB51 for PI3K, p-Akt, and PSD95. Results are mean ± SE of 3 different experiments (n=3). ** p< 0.005, ***p< 0.0005 vs Ctr; ++ p< 0.005, +++ p< 0.0005 vs 6-OHDA. Representative WB figures are shown.
The neuronal death pathway, comprising pro-BDNF, phosphorylated c-Jun N-terminal kinase (p-JNK), phosphorylated extracellular signal–regulated kinase (p-ERK1,2) and P75 was then analyzed. All these proteins were significantly increased by 6-OHDA, while the presence of SLAB51 restored the control conditions, thus confirming a neuroprotective role exerted by the probiotic (Figure 5).
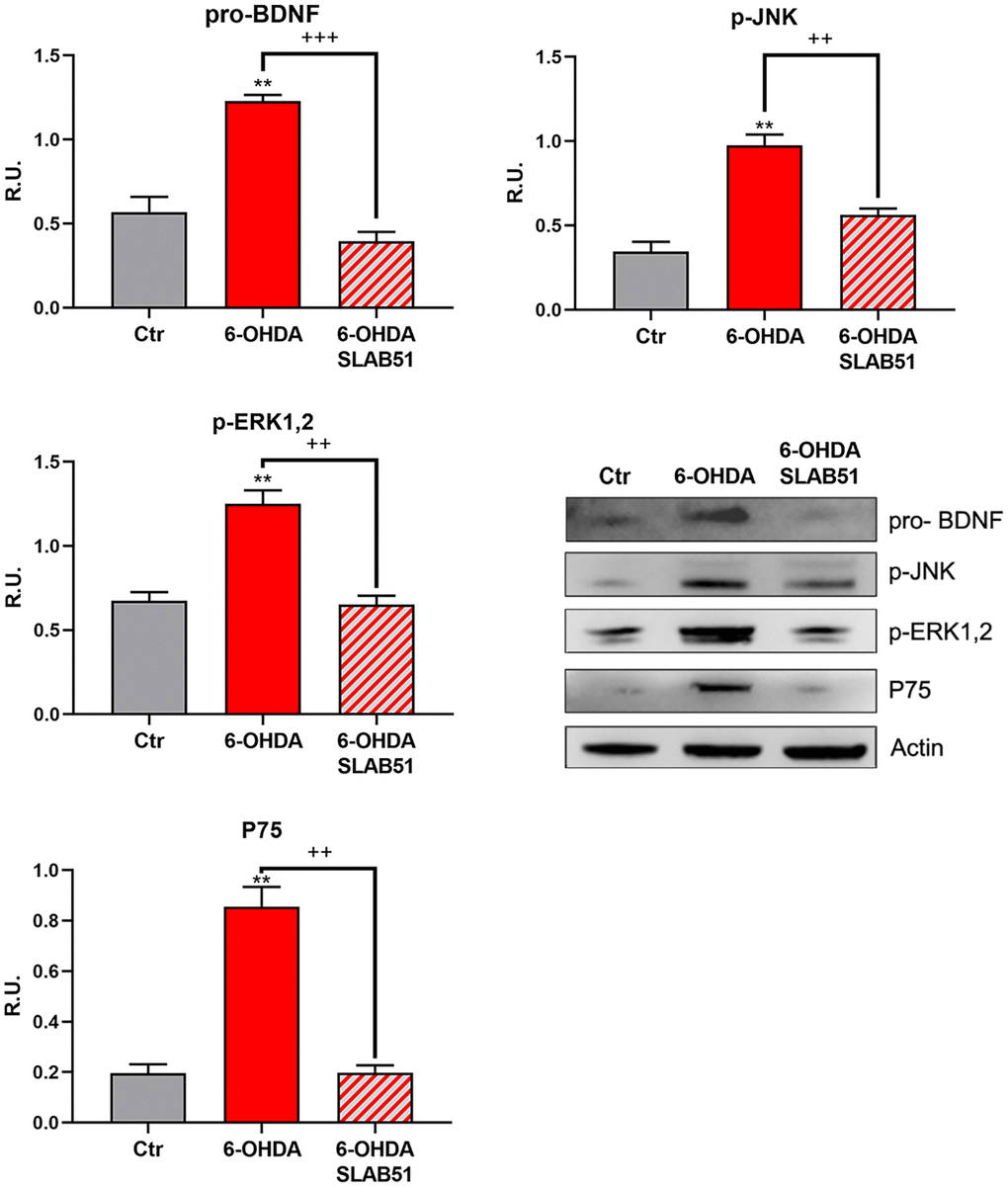
Figure 5. WB and relative densitometric analysis for Ctr, 6-OHDA and 6-OHDA+SLAB51 for pro-BDNF, p-JNK, p-ERK1,2 and P75. Results are mean ± SE of 3 different experiments (n=3). ** p< 0.005 vs Ctr; ++ p< 0.005, +++ p< 0.0005 vs 6-OHDA. Representative WB figures are shown.
Recent evidence reported the involvement of activated peroxisome proliferator activated receptor γ (PPARγ) in modulating BDNF levels in different pathologies, including PD [30]. Thus, on light of the results collected so far, we assayed PPARγ level through Western blotting analysis. Indeed, in 6-OHDA-treated cells a significant reduction of the transcription factor was apparent; while SLAB51 lysate increased PPARγ protein levels (Figure 6). Further, since it is known that a product of lipid peroxidation, 4-hydroxynonenal (4-HNE) is generally increased during oxidative stress, as occur in neurodegeneration processes, and to highlight a potential role of probiotics in counteracting 6-OHDA oxidative injury, 4-HNE protein adducts by Western blotting in in vitro samples were analyzed. It is possible to observe that the probiotic formulation significantly reduced the level of 4-HNE proteins adducts, thus suggesting a protective role of SLAB51 against oxidative damage (Figure 6). Once obtained the above promising results in vitro, in order to evaluate if this formulation was able to modulate neuroprotective pathways in a more complex model, we tested the probiotic in unilateral 6-OHDA-lesioned animal model.
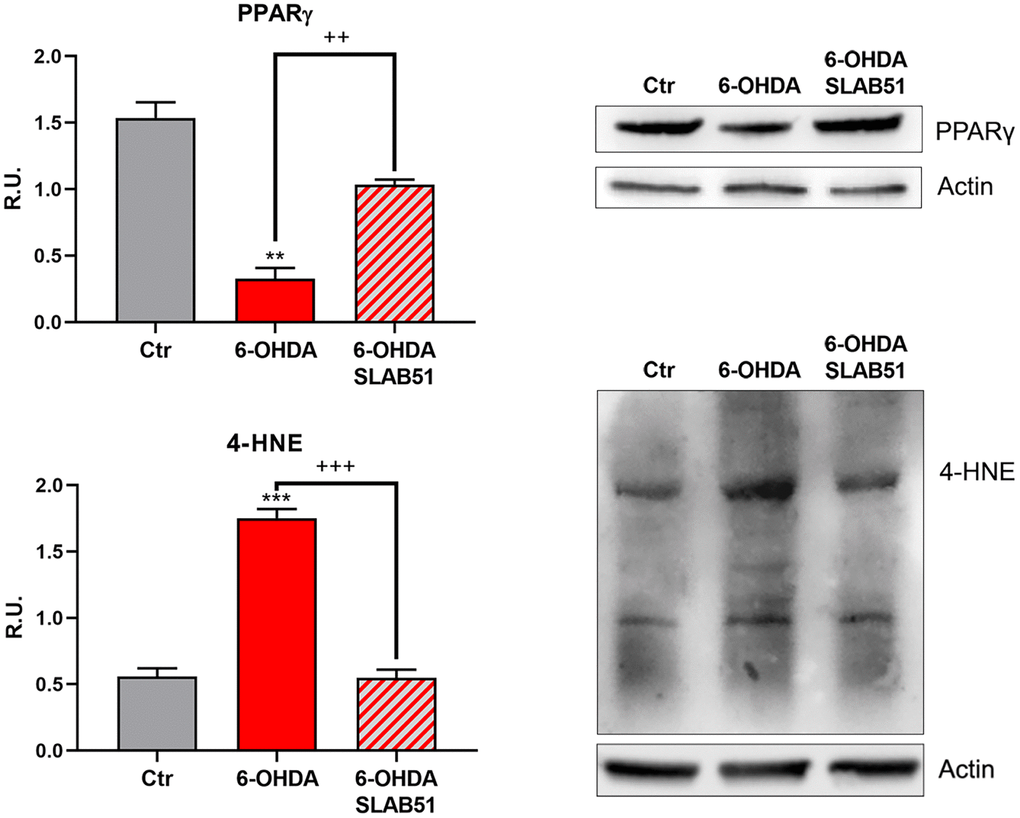
Figure 6. WB and relative densitometric analysis for Ctr, 6-OHDA and 6-OHDA+SLAB51 for PPARγ and 4-HNE proteins adducts. Results are mean ± SE of 3 different experiments (n=3). ** p< 0.005, ***p< 0.0005 vs Ctr; ++ p< 0.005, +++ p< 0.0005, vs 6-OHDA. Representative WB figures are shown.
In vivo
To test the formulation in vivo, SLAB51 diluted in drinking water was daily administered via oral gavage for 2 weeks previous 6-OHDA injection and followed for further 3 weeks (as indicated in “Material and Methods” section and in the timeline Figure 7A, total 5 weeks). Oral administration of the probiotic formulation did not generate neither mortality nor significant variances in the average body weights, in control and treated mice (Figure 7B). In Figure 7B also behavioral tests are shown. Notably, the cylinder test, considered to evaluate locomotor asymmetry in rodent models of CNS diseases, showed that striatum lesion induced a robust and significative decrease in mice motor performance, in particular, it was possible to appreciate a decrease of the use of contralateral paw. Interestingly, SLAB51 was able to counteract behavioral impairment induced by 6-OHDA inoculation, restoring the control conditions.
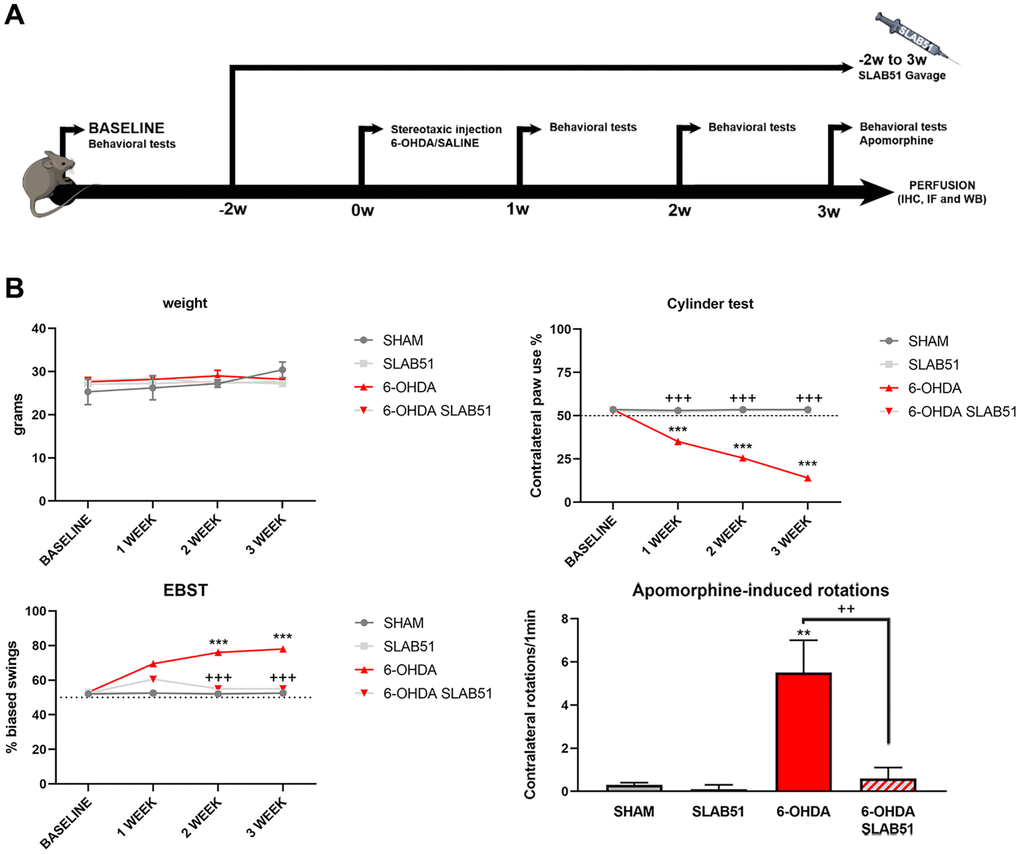
Figure 7. (A) Procedural timeline with specific timepoints. (B) Body weight and behavioral tests in SHAM, SLAB51, 6-OHDA and 6-OHDA+SLAB51 animals. ** p< 0.005, *** p< 0.0005 vs Ctr; ++ p< 0.005, +++ p< 0.0005 vs 6-OHDA.
The Elevated Body Swing Test (EBST), index of asymmetrical motor performance of hemi-parkinsonian models in a drug-free state, in the same figure is shown. 6-OHDA-lesioned mice showed right-biased swings of 70% or greater respect to control animals, while probiotic formulation treatment was able to counteract this effect, in fact, at 2 weeks the animals showed the same behavior of SHAM group. These findings were confirmed by apomorphine test, a tool to monitor motor impairment and functional improvement [31]. In particular, the dopamine (DA) receptor agonist apomorphine (APO), acting post-synaptically and hyperstimulating supersensitive DA receptors in the denervated striatum, leads the animal to rotate in the opposite contralateral direction, i.e., away from the lesioned side. Indeed, in our experimental conditions, upon 6-OHDA mice showed increased contralateral rotations, while the 6-OHDA mice treated with the probiotic formulation had a similar behavior to SHAM groups (Figure 7).
Further, the immunostaining of TH in dopaminergic neurons was performed; it was possible to observe in 6-OHDA-treated animal a marked decrease of TH immunoreactivity, while SLAB51 rescued dopaminergic neurons in both substantia nigra and striatum (CPu) (Figure 8). To further validate these data, immunofluorescence analyses for Dopaminergic Transporter (DAT) in mice substantia nigra were performed. As reported in Figure 9, a massive reduction of DAT fluorescence intensity was observed upon 6-OHDA inoculation (in the right side), while the probiotic formulation counteracted this effect, thus suggesting that SLAB51 exerted neuroprotective activities.
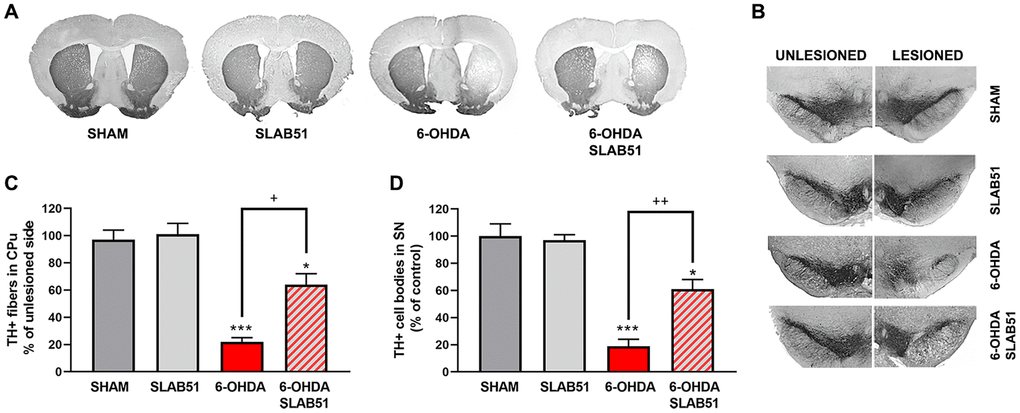
Figure 8. Immunostaining of TH in dopaminergic neurons. Transverse section taken through the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA), immunostained for TH to evaluate the dopaminergic-induced injury by stereotaxic injection of 6-OHDA in the right side. Histograms shows the percentage of TH+ fibers loss in striatum (CPu) and TH+ cell bodies in substantia nigra (SN) (expressed in percentage of unlesioned side). * p< 0.05, *** p< 0.0005 vs Ctr; + p< 0.05, ++ p< 0.005 vs 6-OHDA.
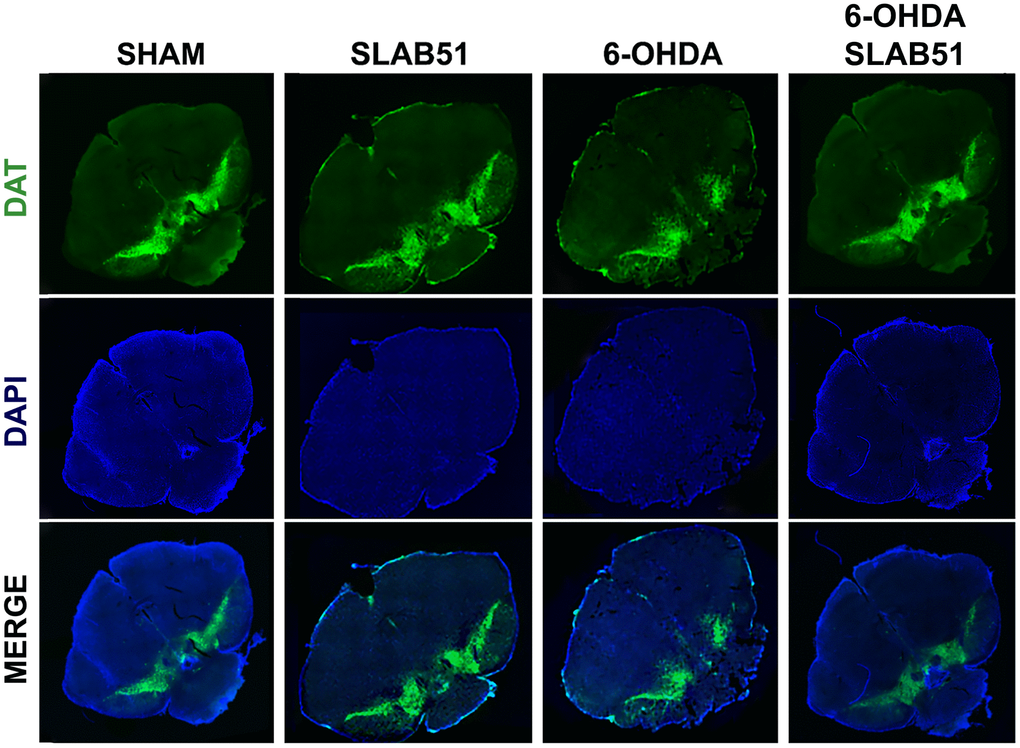
Figure 9. Immunofluorescence for DAT in mice Substantia nigra. Images were taken at confocal microscope at 20x magnification.
Recent studies have demonstrated neuroinflammation and microglia activation in PD [30]. For this reason, immunofluorescence analyses and quantification for the specific marker of microglial activation, microglia ionized calcium-binding adapter molecule 1 (Iba1), and for astrogliosis, glial fibrillary acid protein (GFAP), in brain slices were performed (Figure 10). It is possible to observe a significant increase of Iba1 and GFAP fluorescence intensity in 6-OHDA slices, while SLAB51 was able to counteract 6-OHDA-induced effects, thus indicating a reduction in neuroinflammation.
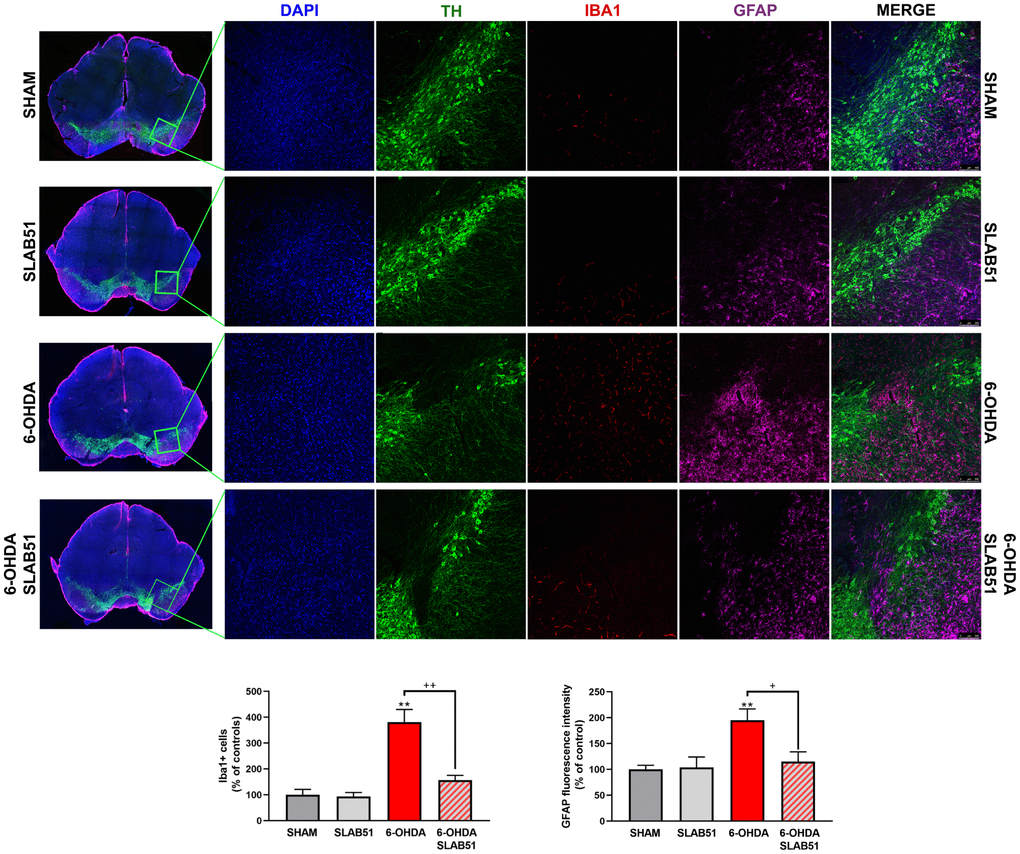
Figure 10. Triple immunostaining at 20x magnification for Iba1, TH and GFAP, nuclei were counterstained with DAPI. On the left it is possible to appreciate mosaic figures, while on the right inset at higher magnification for TH, Iba1 and GFAP staining and merge figures were reported. Histograms for Iba1 show the number of Iba1 + cells, while for GFAP the fluorescence intensity, as % of controls, is plotted. ** p< 0.005 vs Ctr; + p< 0.05, ++ p< 0.005 vs 6-OHDA.
On this basis, PPARγ, a ligand-dependent transcription factor involved in neuroinflammation, oxidative stress and energetic metabolism, that is also able to stimulate neurotrophins release (including BDNF) [30], was analyzed by immunofluorescence in brain slices (Figure 11). Interestingly, SLAB51-treated samples showed the transcription factor at nuclear level, while in 6-OHDA-treated animals, the fluorescence intensity for PPARγ was strongly decreased and, further, localized at cytoplasmic level. Western blotting analyses for PPARγ (Figure 12) demonstrated a restoration of this protein levels in 6-OHDA/SLAB51 treated animals to those of control group. Western blotting results, concomitant with PPARγ localized into the nucleus, may suggest that its activation could be responsible for the reduced neuroinflammation and for the neuroprotection via BDNF pathway, as confirmed by Western Blot analysis performed on in vivo samples. Indeed, BDNF and its receptor TrkB showed the same behavior of PPARγ, suggesting that SLAB51 was able to counteract the toxin-induced lesion both in substantia nigra and striatum (Figure 12).
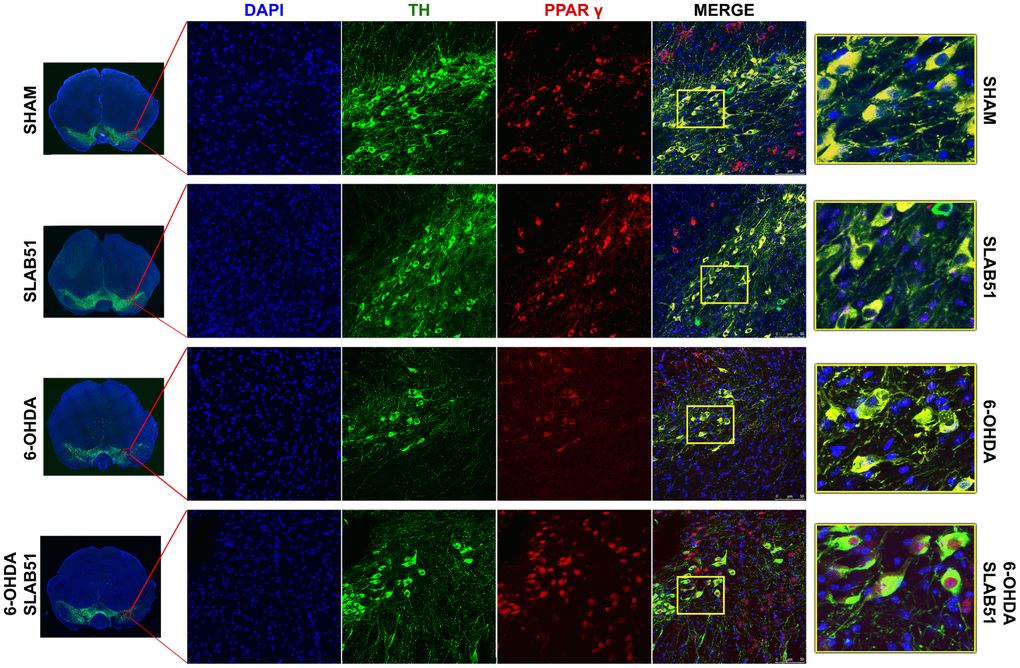
Figure 11. Immunofluorescence analysis for PPARγ in substantia nigra. On the left, the mosaic images obtained using confocal microscopy at 20x magnification were shown. In the center, double immunostaining at 40x magnification with TH and PPARγ as well as the merge figures were reported. On the right it is possible to observe the inset of the indicated boxes.
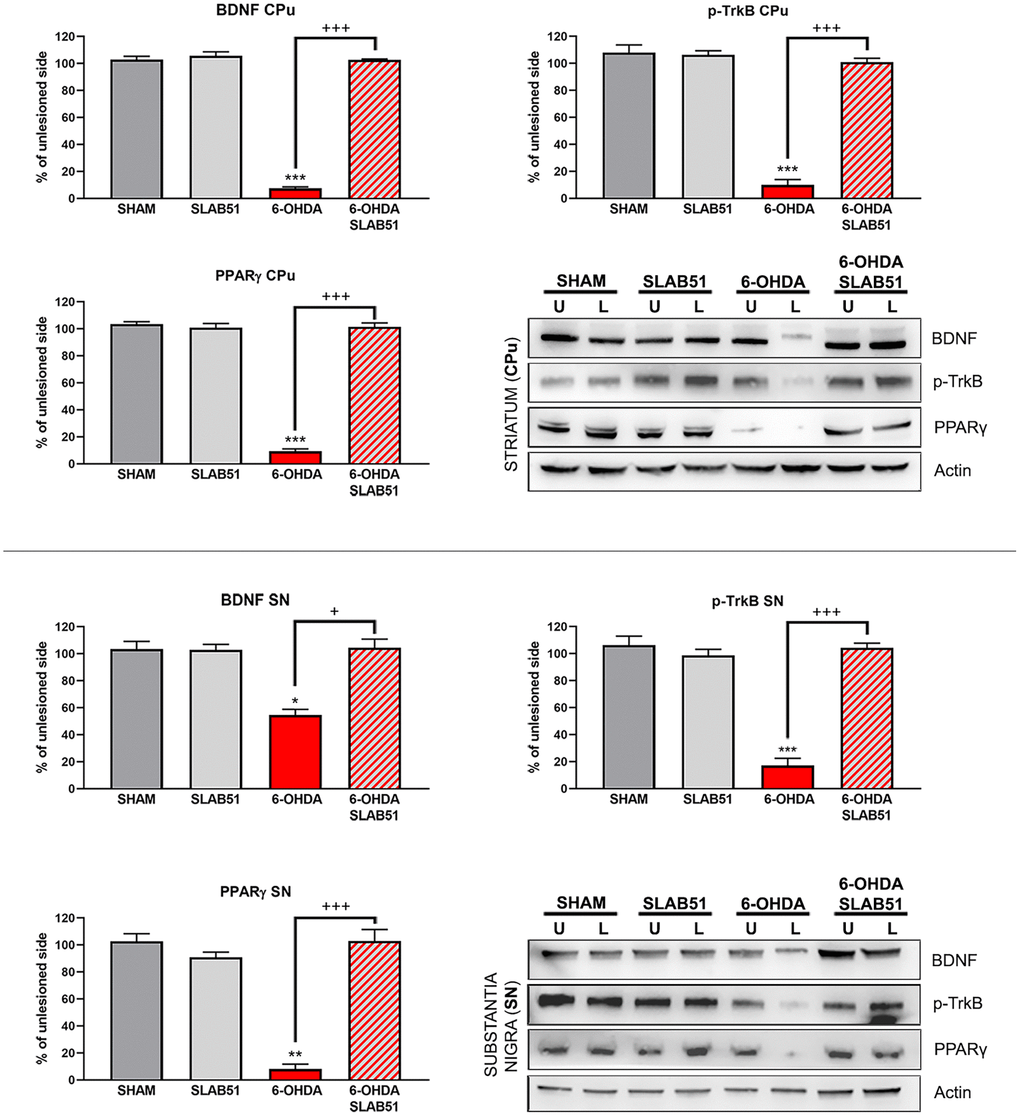
Figure 12. Western blotting and relative densitometric analysis for mBDNF, p-TrkB and PPARγ in substantia nigra (SN) and striatum (CPu). Results are mean ± SE of 3 experiments (n=3). * p< 0.005, ** p< 0.005, *** p< 0.0005 vs Ctr; + p< 0.005, +++ p< 0.0005 vs 6-OHDA. Representative WB images are shown.
Recent findings indicated that hemeoxygenase-1 (HO-1) is regulated by upstream regulators of PPARγ [32]. The antioxidant enzyme HO-1 with established cytoprotective effects has been demonstrated to modulate several pathological processes, including PD. Notably, HO-1 is involved in the release of neurotrophic factors, in the sustainment of dopaminergic neuronal survival in substantia nigra, and in preventing α-synuclein aggregation [33]. Interestingly, in our experimental conditions, 6-OHDA significantly decreased HO-1, while the probiotic formulation was able to counteract 6-OHDA effects, reverting the levels of HO-1 to those of control condition as shown in Figure 13.
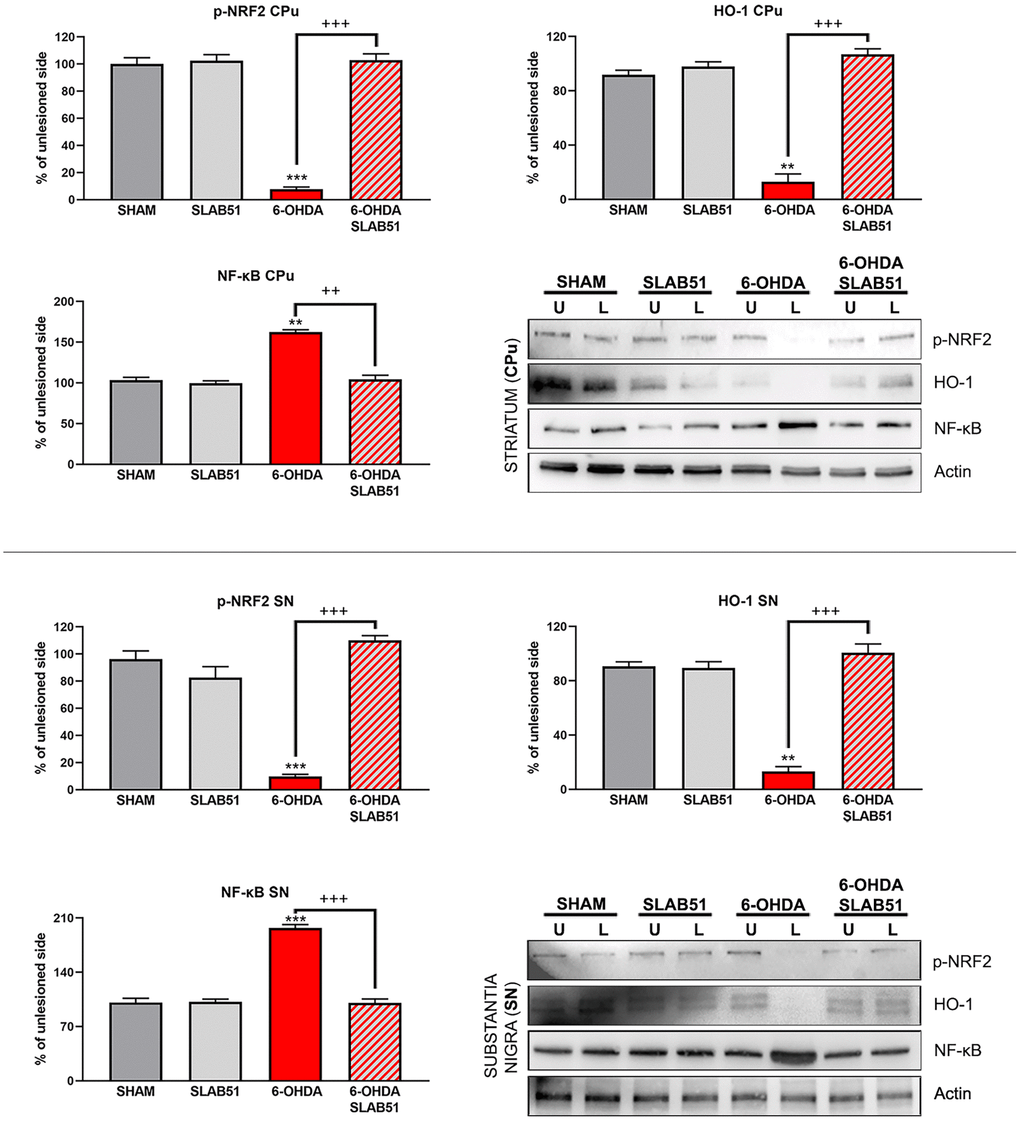
Figure 13. Western blotting and relative densitometric analysis for p-Nfr2, HO-1 and NF-KB in SN and CPu. Results are mean ± SE of 3 experiments (n=3). ** p< 0.005, *** p< 0.0005 vs Ctr; ++ p< 0.0005, +++ p< 0.0005 vs 6-OHDA. Representative WB images are shown.
In addition, nuclear transcription factor-erythroid 2 related factor (Nrf2) is able to bind the antioxidant response element (ARE) present in the HO-1 promoter region [34]. Nrf2 activity decreases with aging and represent one of the main PD risk factors, effectively, the increase of Nrf2 provides protection to dopaminergic neurons by counteracting oxidative stress injury [35]. As shown in Figure 13, p-Nrf2 (transcriptionally active) protein levels were significantly downregulated in 6-OHDA-injured animals, while SLAB51 was able to counteract 6-OHDA effects, both in substantia nigra and striatum.
Further, NRF2-ARE system interacts with nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB), a protein complex involved in cell survival and cytokine release, related to neurodegenerative and neuroinflammatory conditions. In fact, in our experimental conditions, NF-ĸB protein levels were upregulated upon 6-OHDA challenge, while the probiotic treatment reverted this protein to control conditions, both in substantia nigra and striatum (Figure 13), thus suggesting a control in neuronal inflammation and the immune response.
Finally, to confirm the potential pro-survival effect of SLAB51, the apoptosis promotion in dopaminergic neurons of substantia nigra was analyzed by TUNEL assay. As reported in Figure 14, apoptosis dramatically increased in 6-OHDA treatment, while the presence of the probiotic mixture reduced apoptotic nuclei index (to about 10%), thus suggesting that the probiotic formulation protects against 6-OHDA-mediated apoptosis.
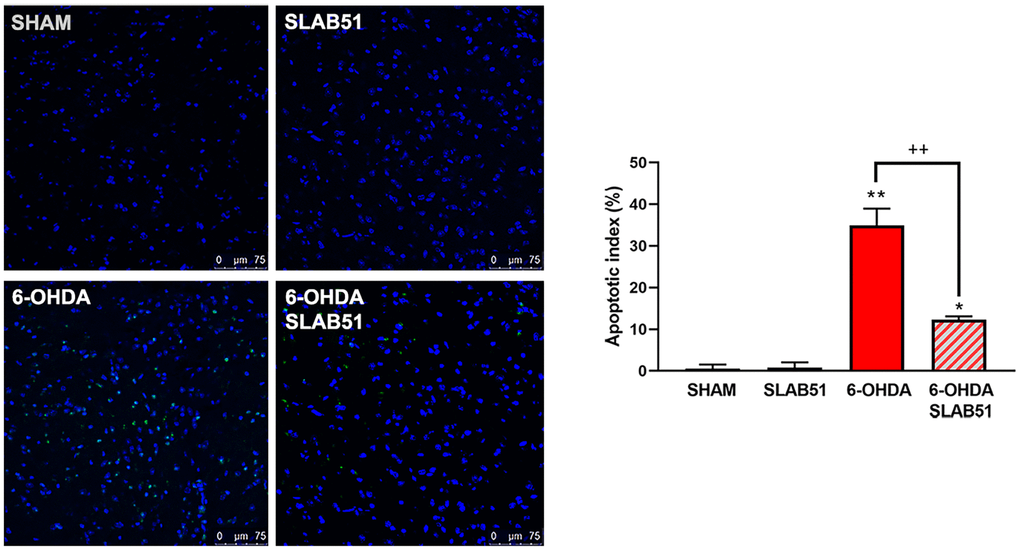
Figure 14. TUNEL assays in mice substantia nigra. Figures were taken at confocal microscope at 40x magnification. The graph shows apoptotic index obtained by counting positive nuclei. * p< 0.005, ** p< 0.005 vs ctr; ++< 0.005 vs 6-OHDA. Representative figures are reported.