Cancer stem cell (CSC) based mitochondrial signatures for predicting distant metastasis and tumor recurrence
After a breast cancer diagnosis, most patients undergo surgical resection of the primary tumor and are then subsequently treated with hormone-, chemo- and/or radio-therapy, depending on the breast cancer subtype. However, many patients ultimately experience treatment failure, resulting in tumor recurrence and distant metastasis. Unfortunately, distant metastasis is responsible for the premature deaths in the vast majority of cancer patients, approaching >90% (Figure 1). Therefore, new diagnostics and therapeutics are urgently needed to prevent and treat metastatic disease, which has been attributed to the existence and resurgence of a small sub-population of cancer cells, known as cancer stem cells (CSCs).
Figure 1. Clinical course of cancer therapy: Focus on the causes of treatment failure. After diagnosis, breast cancer patients undergo surgical resection of the primary tumor and then are treated with a specific therapy (hormone/chemo/radio), depending on the breast cancer subtype and clinical staging. However, a significant number of patients ultimately undergo treatment failure, resulting in tumor recurrence and distant metastasis. Distant metastasis is responsible for the premature deaths of >90% of cancer patients, undergoing treatment failure. This phenomenon has been attributed to the propagation and dissemination of CSCs.
In order to identify new molecular targets that are selectively up-regulated in CSCs, we previously carried out unbiased proteomics analysis on MCF7 cell 2D-monolayers, as directly compared with MCF7 3D-mammospheres, which are known to be highly enriched in CSCs and progenitor cells [6]. As a consequence, we observed that 25 mitochondrial proteins were highly up-regulated by >100-fold, specifically in 3D-mammospheres [6].
Here, we interrogated whether the mRNA transcripts of these mitochondrial proteins show any prognostic value in large numbers of ER(+) human breast cancer patients. Interestingly, we observed that 13 of these 25 gene transcripts showed prognostic value in predicting distant metastasis. We then used these 13 gene transcripts to create a mitochondrial-related gene signature, that effectively predicted distant metastasis in 1,395 patients (HR=1.79; P=3.4e-07). See Supplementary Table 1 and Figure 2.
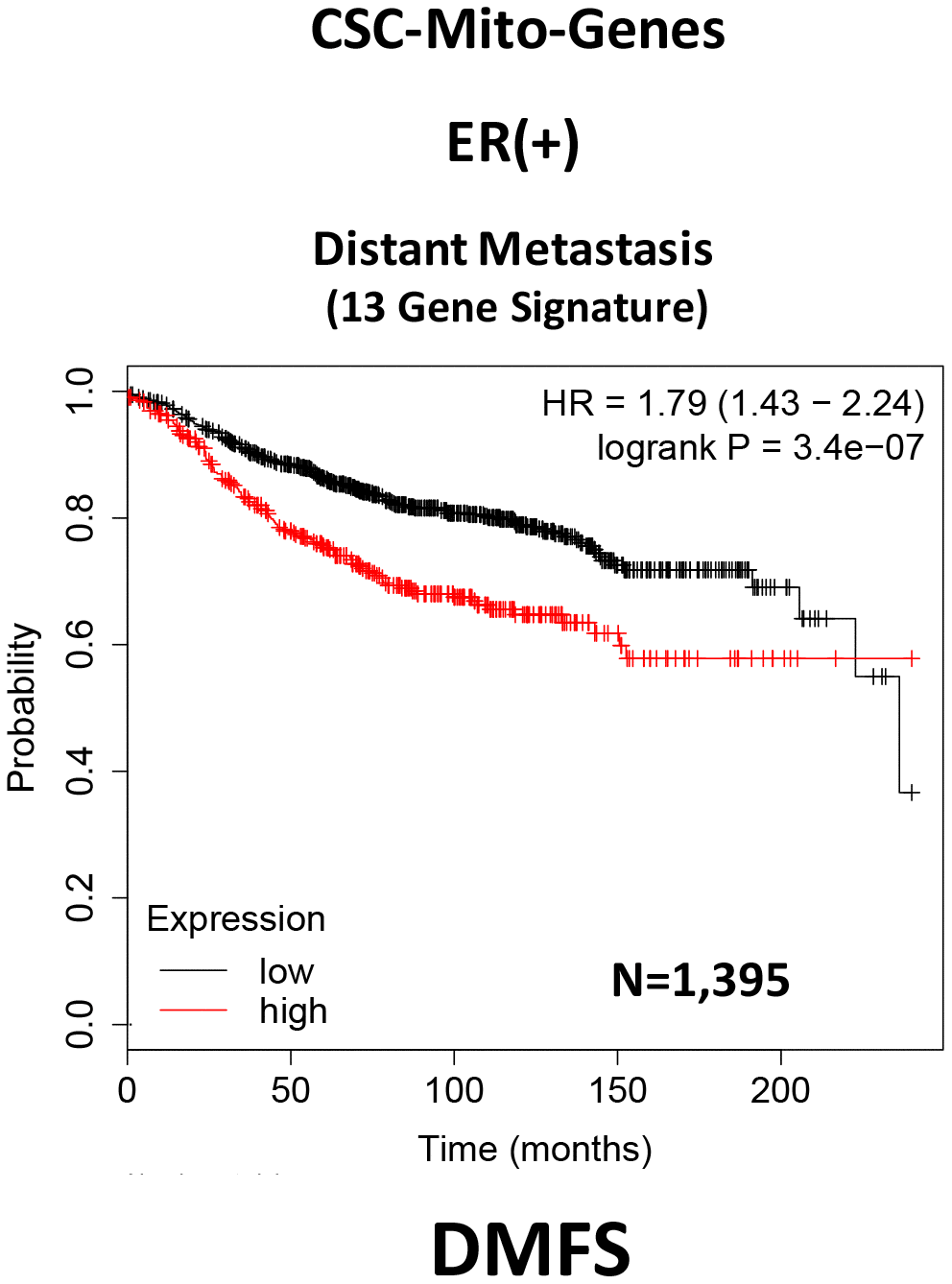
Figure 2. A CSC-based mitochondrial 13-gene signature predicts distant metastasis in ER(+) breast cancer patients. We used 13 gene transcripts to create a CSC-based mitochondrial-related gene signature, that effectively predicted distant metastasis in N=1,395 patients (HR=1.79; P=3.4e-07). See also Supplementary Table 1.
To optimize its predictive value, we next selected the top 4 gene transcripts, with the largest hazard ratios, to construct a short 4-gene signature, which revealed an increase in prognostic value, related to distant metastasis (HR=1.91; P=2.2e-08). Remarkably, this 4-gene signature was also able to predict tumor recurrence in the same patient population (HR=1.68; P=1.2e-15; Supplementary Table 2 and Figure 3).
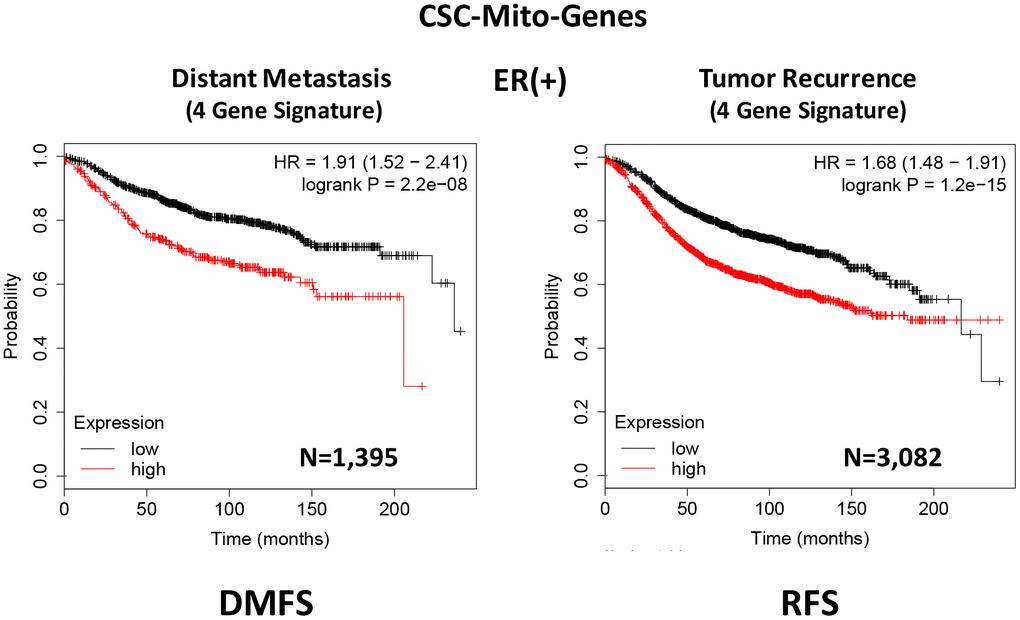
Figure 3. A CSC-based mitochondrial 4-gene signature predicts distant metastasis and tumor recurrence in ER(+) breast cancer patients. To optimize its predictive value, we constructed a short 4-gene signature, which revealed an increase in prognostic value, related to distant metastasis (HR=1.91; P=2.2e-08). This 4-gene signature was also able to predict tumor recurrence in the same patient population (HR=1.68; P=1.2e-15). See also Supplementary Tables 2 and 3 and 3. Therefore, these CSC-based mitochondrial signatures may provide a new prognostic approach for predicting treatment failure in breast cancer patients. DMFS, distant metastasis free survival; RFS, relapse free survival.
Therefore, we conclude that these CSC-based mitochondrial signatures may provide a new prognostic approach for predicting distant metastasis and tumor recurrence in breast cancer patients. Most importantly, these results may also biologically and functionally implicate CSC mitochondria in the process of metastasis and tumor recurrence.
Mitochondrial inhibitors metabolically target and prevent cancer cell metastasis, without significant toxicity
To functionally evaluate the role of mitochondria in cancer metastasis, we used a series of mitochondrial inhibitors that were previously developed to specifically target the propagation of CSCs, known as the Mitoriboscins [15]. These inhibitors were developed via in silico screening of a library of 45,000 compounds, to identify positive hits that bound to the 3D-structure of the large mitochondrial ribosome [15]. After 880 positive hits were identified, these compounds were then subjected to phenotypic drug screening, using an ATP-depletion assay, and directly validated using the Seahorse Metabolic Flux analyser, to confirm their specificity as bonafide mitochondrial inhibitors [15]. Ultimately, this screening approach led to the identification of three major compounds, known as 23/G4, 24/D4 and 24/F9, which all inhibited 3D-mammosphere formation in MCF7 cells and significantly blocked cell migration in MDA-MB-231 cells, all in the low micro-molar range [15]. The structures of 23/G4, 24/D4 and 24/F9 are shown in Figure 4.
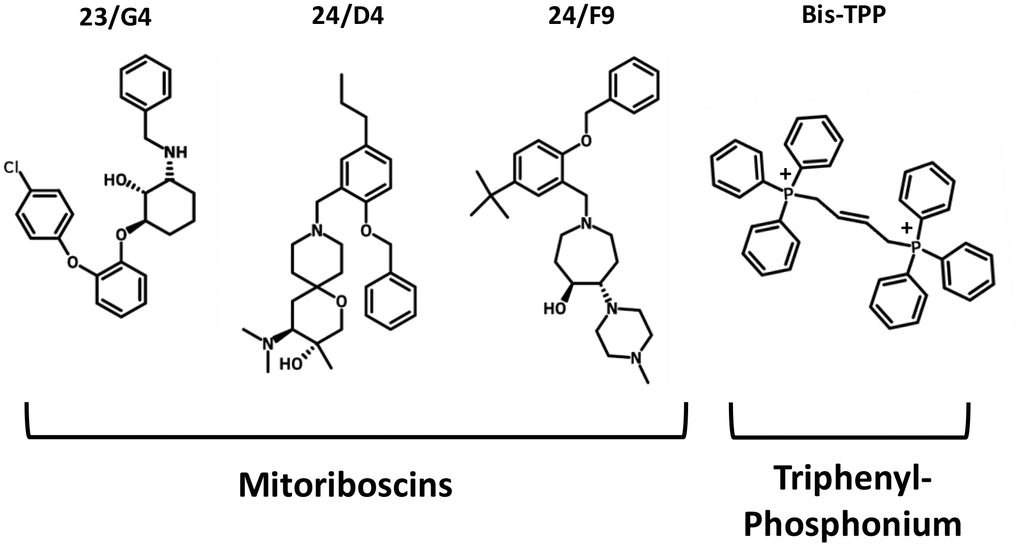
Figure 4. Mitochondrial inhibitors: Mitoriboscins and bis-1,4-butene-TPP. The chemical structures of the three Mitoriboscins (23/G4, 24/D4 and 24/F9) and Bis-TPP are shown.
To experimentally evaluate their functional effects in vivo, we used MDA-MB-231 cells and the well-established chorio-allantoic membrane (CAM) assay in chicken eggs, to quantitatively measure tumor growth and distant metastasis. An inoculum of 1 X 106 MDA-MB-231 cells was added onto the CAM of each egg (day E9) and then eggs were randomized into groups. On day E10, tumors were detectable and they were then treated daily for 8 days with vehicle alone (1% DMSO in PBS) or the three Mitoriboscin compounds. In parallel, we also evaluated the activity of another mitochondrial inhibitor, namely butane-1,4-bis-triphenyl-phosphonium (Bis-TPP), which we identified as an inhibitor of 3D-mammosphere formation in MCF7 cells, with an IC-50 of less than 0.5 μM [16]. It is well-established that the TPP-moiety acts as a chemical signal for mitochondrial targeting [16, 17].
After 8 days of drug administration, on day E18 all tumors were weighed, and the lower CAM was collected to evaluate the number of metastatic cells, as analyzed by qPCR with specific primers for Human Alu sequences.
Figure 5 shows the effects of the three Mitoriboscins (23/G4, 24/D4, 24/F9) and Bis-TPP on MDA-MB-231 tumor growth. Note that none of the four inhibitors tested showed any significant effects on tumor growth, as a result of the 8-day period of drug administration.
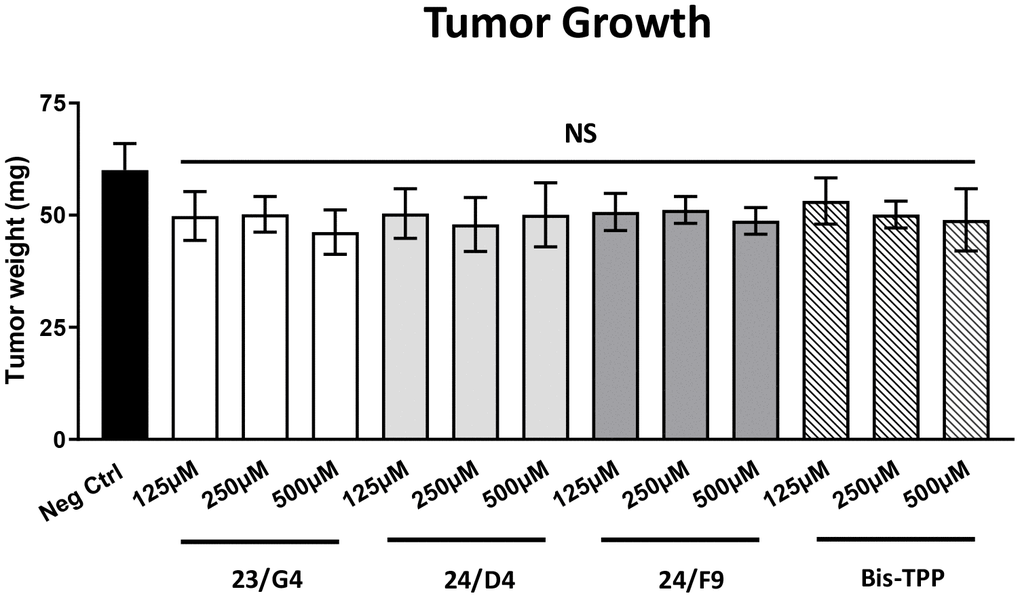
Figure 5. Mitochondrial inhibitors have no effect on tumor growth. MDA-MB-231 cells and the well-established chorio-allantoic membrane (CAM) assay in chicken eggs were used to quantitatively measure tumor growth. An inoculum of 1 X 106 MDA-MB-231 cells was added onto the CAM of each egg (on Day E9) and then eggs were then randomized into groups. On day E10, tumors were detectable and they were then treated daily for 8 days with vehicle alone (1% DMSO in PBS) or the four mitochondrial inhibitors. After 8 days of drug administration, on day E18 all tumors were weighed. Note that none of the mitochondrial inhibitors tested had any significant effects on tumor growth. Averages are shown + SEM. NS, not significant.
However, all four mitochondrial inhibitors showed significant effects on MDA-MB-231 cancer cell metastasis. Figure 6 illustrates that all three Mitoriboscins were clearly effective in inhibiting metastatic progression, although 24/D4 and 24/F9 were the most effective. In addition, Bis-TPP also significantly prevented metastasis.
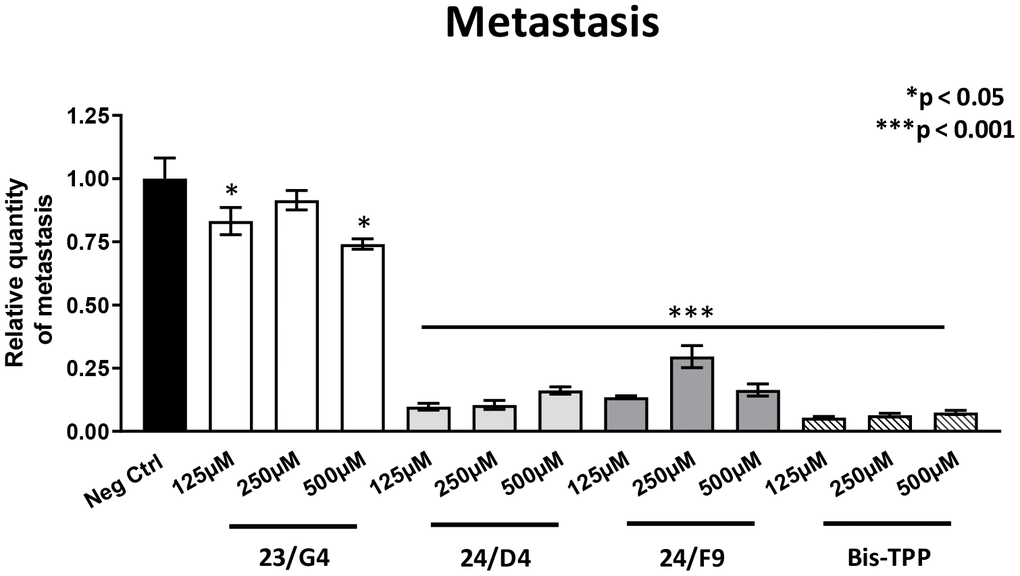
Figure 6. Mitochondrial inhibitor compounds selectively target and prevent cancer metastasis. MDA-MB-231 cells and the well-established chorio-allantoic membrane (CAM) assay in chicken eggs were used to quantitatively measure spontaneous tumor mestastasis. An inoculum of 1 X 106 MDA-MB-231 cells was added onto the CAM of each egg (on day E9) and then eggs were then randomized into groups. On day E10, tumors were detectable and they were then treated daily for 8 days with vehicle alone (1% DMSO in PBS) or the four mitochondrial inhibitors. After 8 days of drug administration, the lower CAM was collected to evaluate the number of metastatic cells, as analyzed by qPCR with specific primers for Human Alu sequences. Note that all four mitochondrial inhibitors did show significant effects on MDA-MB-231 metastasis. More specifically, all three Mitoriboscins were clearly effective in inhibiting metastasis, although 24/D4 and 24/F9 were the most effective. In addition, Bis-TPP also significantly prevented metastasis. Averages are shown + SEM. *p<0.05; ***p<0.001.
As 23/G4 was minimally effective at a concentration of 0.5 mM, we also tested it at higher concentrations of 0.75, 1 and 2 mM. Importantly, our results show that 23/G4, at these concentrations, significantly inhibited both tumor growth (by 40% to 60%; Figure 7) and metastatic progression (by 70-75%; Figure 8). Interestingly, as expected, the effects of 23/G4 on metastasis were significantly more pronounced.
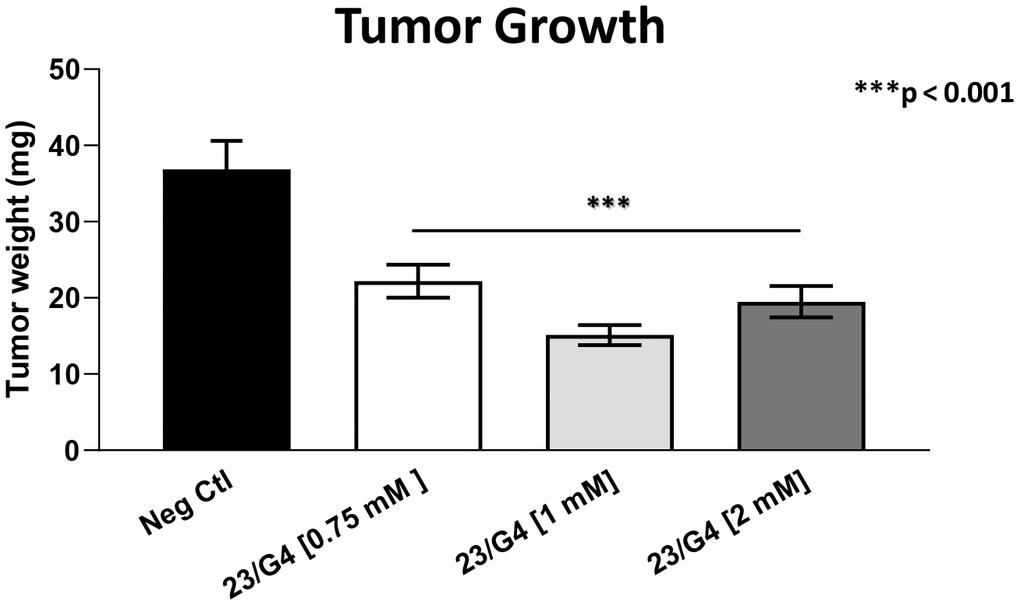
Figure 7. Effects of the Mitoriboscin 23/G4 on tumor growth. The Mitoriboscin 23/G4 was tested at higher concentrations of 0.75, 1 and 2 mM. Note that 23/G4, at these concentrations, inhibited tumor growth (by 40% to 60%). Averages are shown + SEM. ***p<0.001.
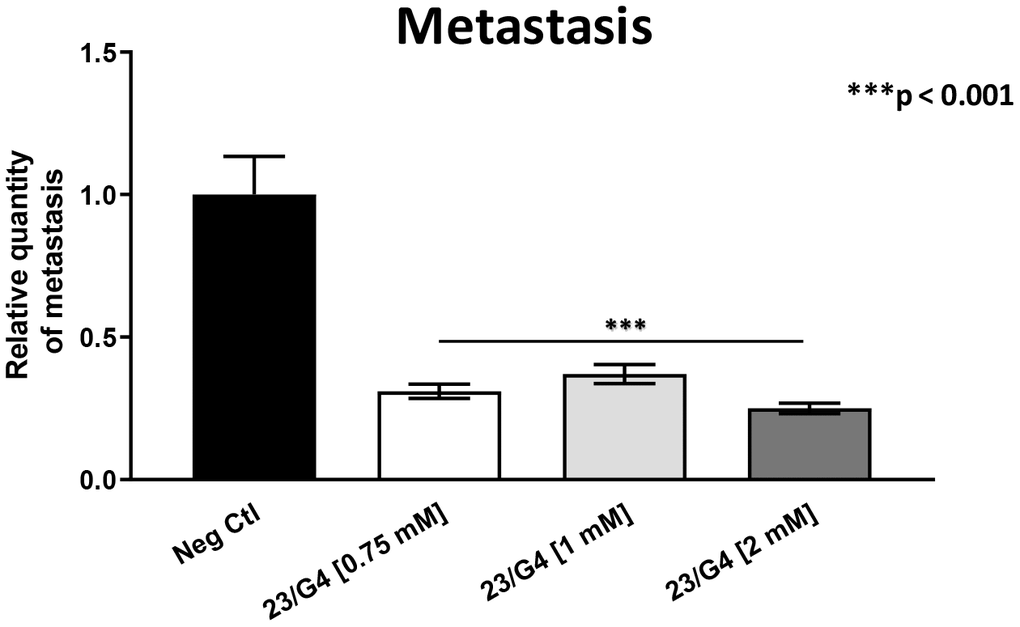
Figure 8. Effects of the Mitoriboscin 23/G4 on cancer metastasis. The Mitoriboscin 23/G4 was tested at higher concentrations, namely 0.75, 1 and 2 mM. Note that 23/G4, at these concentrations, significantly inhibited metastasis (by 70-75%). Interestingly, the effects of 23/G4 on metastasis were significantly more pronounced than its effects on tumor growth. Averages are shown + SEM. ***p<0.001.
Remarkably, in this series of experiments, little or no embryo toxicity was observed, otherwise tumor growth and cancer metastasis assays could not have been completed (Tables 1–3). Therefore, we conclude that mitochondrial inhibitors can be used experimentally, to preferentially inhibit the initiation of tumor metastasis, without significant toxicity.
Table 1. Chick embryo toxicity of Mitoriboscins and Bis-TPP at a concentration of 0.5 mM.
| Group # | Group Description | Total | Alive | Dead | % Alive | % Dead |
| 1 | Neg. Ctrl. | 18 | 16 | 2 | 88.89 | 11.11 |
| 2 | 23/G4 | 10 | 7 | 3 | 70 | 30 |
| 3 | 24/D4 | 12 | 12 | 0 | 100 | 0 |
| 4 | 24/F9 | 10 | 8 | 2 | 80 | 20 |
| 5 | Bis-TPP | 12 | 7 | 5 | 58.33 | 41.67 |
Table 2. Chick embryo toxicity of Mitoriboscin 23/G4 at higher concentrations.
| Group # | Group Description | Total | Alive | Dead | % Alive | % Dead |
| 1 | Neg Ctrl | 17 | 12 | 5 | 70.59 | 29.41 |
| 2 | 23/G4 [0.75 mM] | 14 | 10 | 4 | 71.43 | 28.57 |
| 3 | 23/G4 [1 mM] | 15 | 12 | 3 | 80 | 20 |
| 4 | 23/G4 [2 mM] | 15 | 10 | 5 | 66.67 | 33.33 |
Table 3. Chick embryo toxicity of Dodecyl-TPP.
| Group # | Group Description | Total | Alive | Dead | % Alive | % Dead |
| 1 | Neg. Ctrl. | 18 | 13 | 5 | 72.22 | 27.78 |
| 2 | d-TPP [6.25 μM] | 19 | 13 | 6 | 68.42 | 31.58 |
| 3 | d-TPP [25 μM] | 19 | 13 | 6 | 68.42 | 31.58 |
| 4 | d-TPP [62.5 μM] | 19 | 3 | 16 | 15.79 | 84.21 |
Finally, we also tested another more potent mitochondrially-targeted TPP compound, namely Dodecyl-TPP, using low micro-molar concentrations (6.25- and 25-μM). Figures 9 and 10 demonstrate that Dodecyl-TPP significantly inhibited tumor growth (by 12% to 40%; Figure 9) and metastatic progression (by 25 to 65%; Figure 10). As predicted, Dodecyl-TPP preferentially targeted metastasis, rather than tumor growth. It is worth noting that Dodecyl-TPP showed some toxicity, but only at 62.5-μM, preventing reliable analysis of its effects on tumor growth and metastasis, at this higher concentration (Table 3). However, Dodecyl-TPP showed little or no toxicity at 6.25- and 25-μM (Table 3).
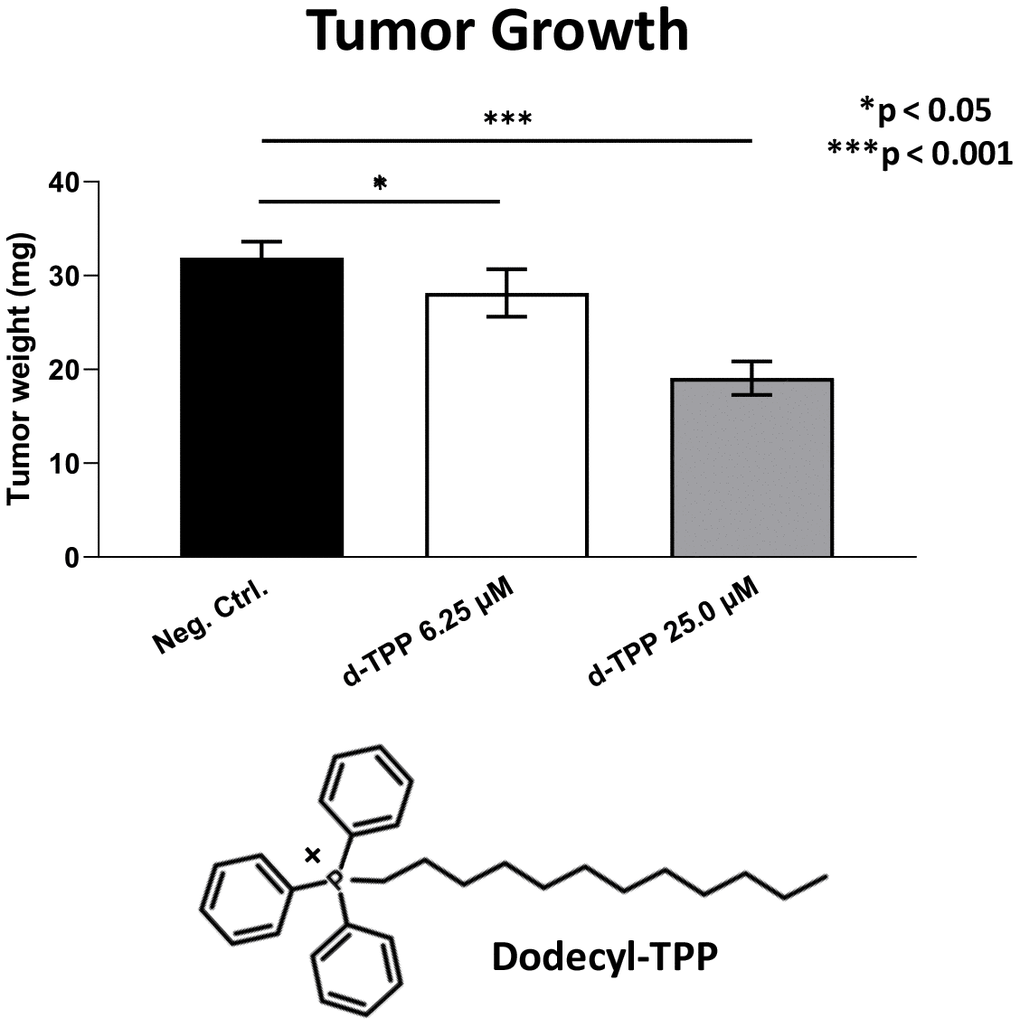
Figure 9. Effects of Dodecyl-TPP on tumor growth. Dodecyl-TPP, another more potent mitochondrially-targeted TPP compound, was tested using low micro-molar concentrations (6.25- and 25-μM). Note that Dodecyl-TPP significantly inhibited tumor growth (by 12% to 40%). Averages are shown + SEM. *p<0.05; ***p<0.001. The structure of Dodecyl-TPP (d-TPP) is also shown. Note the 12-carbon alkyl-chain attached to the lipophilic cation, triphenyl-phosphonium (TPP).
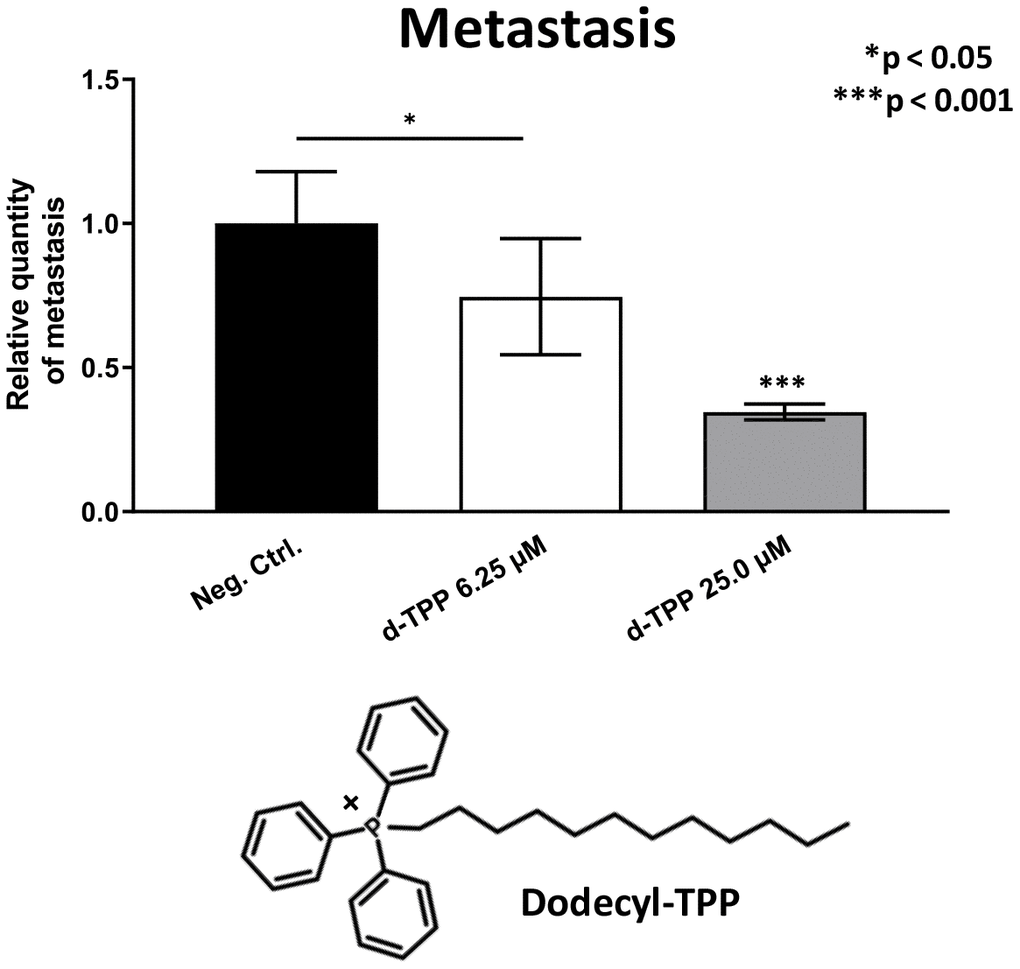
Figure 10. Effects of Dodecyl-TPP on cancer metastasis. Dodecyl-TPP was tested using low micro-molar concentrations (6.25- and 25-μM). Note that Dodecyl-TPP significantly inhibited metastasis (by 25% to 65%). Averages are shown + SEM. *p<0.05; ***p<0.001. Importantly, little or no toxicity was observed for Dodecyl-TPP at 6.25- and 25-μM (Table 3). The structure of Dodecyl-TPP (d-TPP) is also shown.
Mito-Ribosome based signatures for predicting distant metastasis and tumor recurrence: implications as companion diagnostics
Given the functional effects of the Mitoriboscin compounds on metastasis, we next evaluated if the gene mRNA transcripts of the large mitochondrial ribosomal proteins (MRPL) show any prognostic value in ER(+) and ER(-)/basal breast cancer patients.
In ER(+) breast cancer, a 9-gene mito-ribosome signature was able to effectively predict distant metastasis in N=1,395 patients (HR=1.59; P=5e-05) and tumor recurrence in N=3,082 patients (HR=1.71; P<1e-16) (See Supplementary Tables 4 and 5; Figure 11). Importantly, a closely related mito-ribosome signature was also able to predict treatment failure in a sub-set of ER(+) patients undergoing Tamoxifen treatment, which resulted in distant metastasis (N=618 patients; HR=2.16; P=1.7e-05) and tumor recurrence (N=799 patients; HR=3.45; P=1.6e-08) (Supplementary Tables 6 and 7; Figure 12).
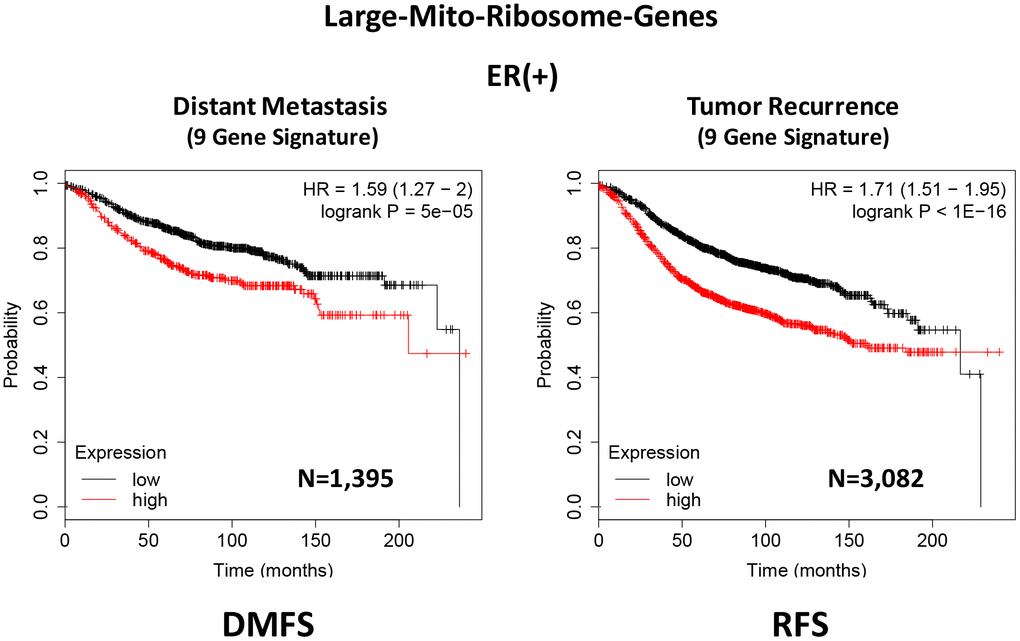
Figure 11. A large mito-ribosome 9-gene signature predicts distant metastasis and tumor recurrence in ER(+) breast cancer patients. A 9-gene mito-ribosome signature effectively predicts distant metastasis in N=1,395 patients (HR=1.59; P=5e-05) and tumor recurrence in N=3,082 patients (HR=1.71; P<1e-16). See Supplementary Tables 4 and 5 and 5 DMFS, distant metastasis free survival; RFS, relapse free survival.
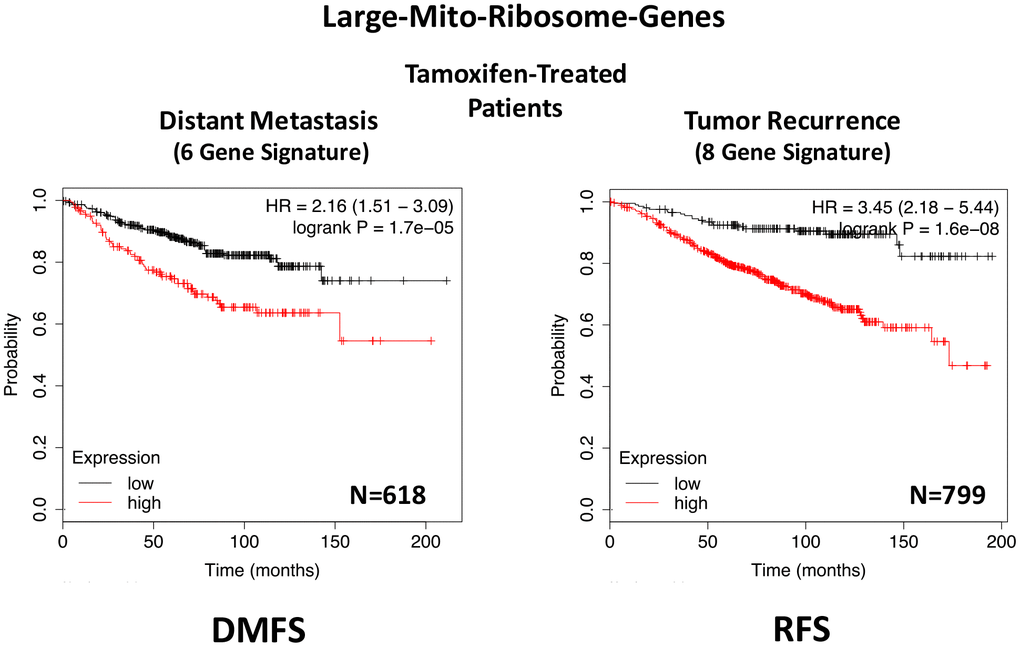
Figure 12. A large mito-ribosome gene signature predicts distant metastasis and tumor recurrence in ER(+) breast cancer patients, treated with Tamoxifen. A mito-ribosome signature predicts treatment failure in a sub-set of ER(+) patients undergoing Tamoxifen treatment, which resulted in distant metastasis (N=618 patients; HR=2.16; P=1.7e-05) and tumor recurrence (N=799 patients; HR=3.45; P=1.6e-08). See also Supplementary Tables 6 and 7 and . DMFS, distant metastasis free survival; RFS, relapse free survival.
In ER(-)/basal breast cancer, a distinct 6-gene mito-ribosome MRPL signature was also able to effectively predict distant metastasis in N=145 patients (HR=2.95; P=0.0018) and tumor recurrence in N=360 patients (HR=2.19; P=1.9e-06), as well as overall survival in N=153 patients (HR=3.17; P=0.00033) (Supplementary Table 8; Figures 13 and 14).
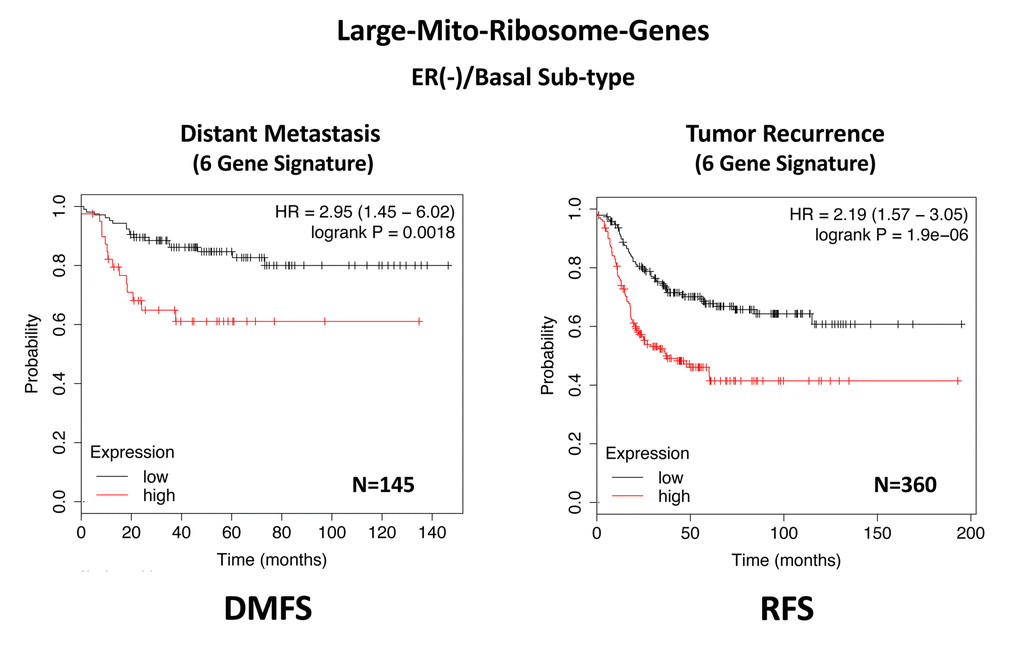
Figure 13. A large mito-ribosome gene signature predicts distant metastasis and tumor recurrence in ER(-)/basal breast cancer patients. In ER(-)/basal breast cancer, a 6-gene mito-ribosome signature was also able to effectively predict distant metastasis in N=145 patients (HR=2.95; P=0.0018) and tumor recurrence in N=360 patients (HR=2.19; P=1.9e-06). See also Supplementary Table 8.
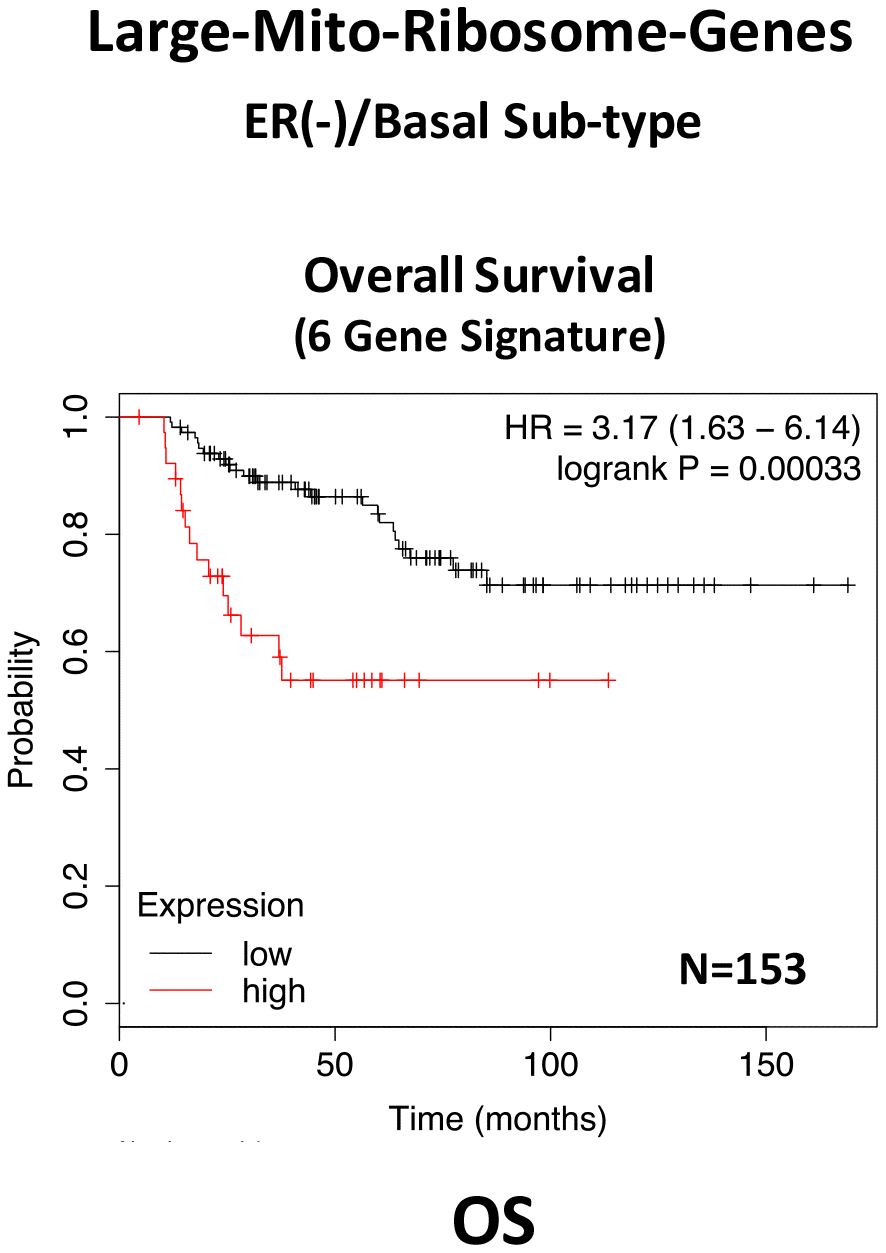
Figure 14. A large mito-ribosome gene signature predicts overall survival in ER(-)/basal breast cancer patients. In ER(-)/basal breast cancer, a 6-gene mito-ribosome signature was also able to effectively predict overall survival in N=153 patients (HR=3.17; P=0.00033).
In summary, these short mito-ribosome gene signatures may also be useful as companion diagnostics to assess which patient populations may benefit most from the administration of the Mitoriboscin compounds.