Introduction
Ovarian cancer (OC) is the eighth-leading cause of cancer-related deaths amongst women. In 2018, over 295,000 new cases, and 180,000 deaths were OC-associated [1]. Invasive epithelial ovarian cancer (EOC) is classified in five prevalent subtypes that originate from both secretory epithelial cells of the distal fallopian tube or from the ovarian epithelium and other tissues [2–7]. Mutational analysis of EOC samples has subdivided the serous OC in type I (less aggressive tumors, including low-grade serous, endometrioid, mucinous, and clear cells carcinomas, mutated for KRAS, BRAF and PI3K) and type II (highly aggressive tumors characterized by TP53 mutation [8–13], and high incidence of double-strand DNA break repair pathways [13–16]. EOC features justify, at least partially, the initial satisfactory response to platin and taxane derivate compounds therapy. Nonetheless, approximately 80% of the patients experience disease recurrence due to chemoresistance [17].
Chemokines secreted in tumor microenvironment (TME), as CXCL2 and CXCL8, correlate to chemoresistance [18–21]. In agreement, cDNA microarray data obtained from NAC-1 knockdown (KD) in high grade serous ovarian cancer (HGSOC) cells, a molecule crucial for the disease development and progression, revealed CXCL2 and CXCL8 secretion [18]. Moreover, these chemokines exert autocrine effect on OC cells by binding to CXCR2 and promoting chemoresistance (manuscript in preparation). CXCR2 activation is associated with cell proliferation, angiogenesis, metastasis and chemoresistance in melanoma, colon, lung and ovarian cancers [19–24].
Herein, we have shown that CXCR2 is crucial for the acquisition of cisplatin chemoresistant phenotype by OC cells, hence introducing a novel potential target against OC.
Results
OC pan resistant ACRP cells were generated from the parental sensitive lineage A2780
ACRP cells were generated from its parental counterpart A2780 lineage, following chronic exposure to cisplatin (1μM to 10 μM). MTT method was used to estimate IC50 for cisplatin, paclitaxel and doxorubicin that were 3.64-fold (p<0.005) (Figure 1A), 77.27-fold (p<0.001) (Figure 1B), and 21.42-fold (p<0.001) (Figure 1C) higher in ACRP than in A2780, respectively, thus proving that the ACRP has emerged as a pan-resistant lineage. Cross-resistance to antineoplastic drugs, as observed in our in vitro study model, supports the need to elucidate the mechanisms of chemoresistance acquisition by OC cells and chemotherapy failure.
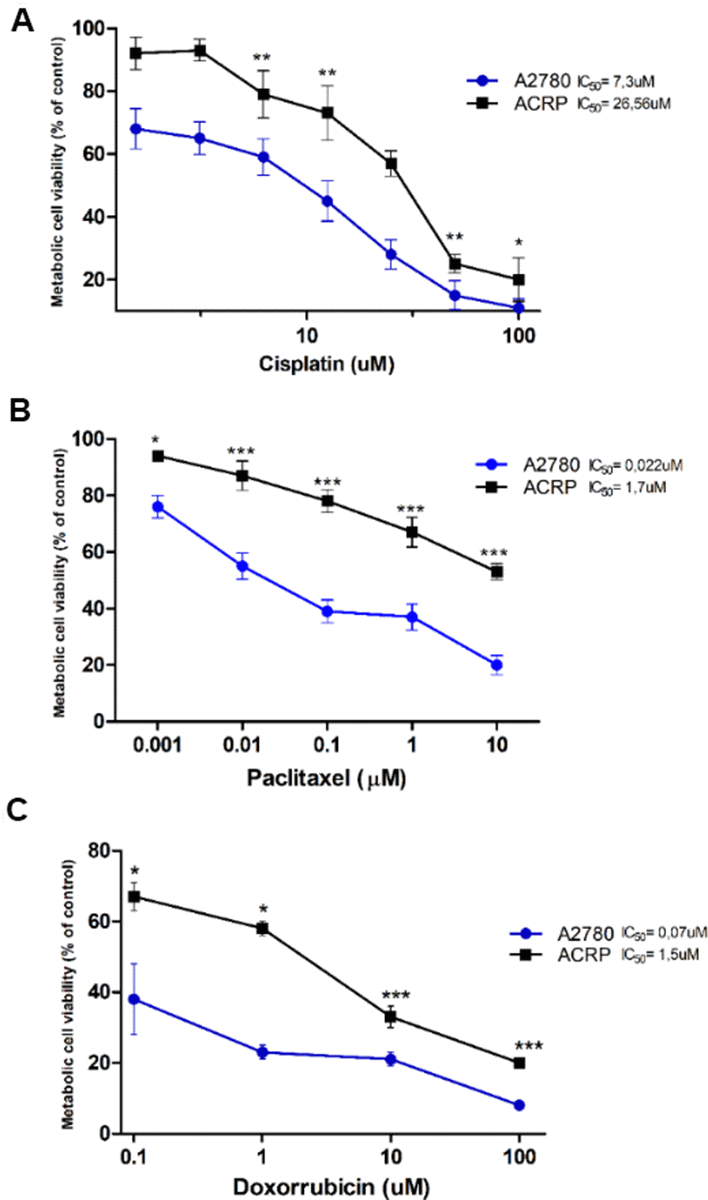
Figure 1. Generation of a pan-resistant ovarian cancer (OC) cells (ACRP) from its parental sensitive counterpart (A2780). (A) ACRP is 3.64-fold more resistant to cisplatin (1.5uM - 100uM) than A2780. (B) ACRP is 77.27-fold more resistant to paclitaxel (1 nM - 10 μM) than A2780. (C) ACRP is 21.42-fold more resistant to doxorubicin (0.1uM - 100uM) than A2780. Estimated IC50 for drugs in tested lineages were calculated by MTT assay, following 24h of cells treatment with each drug within the aforementioned concentration ranges of drugs, which correlate to their circulating concentration in EOC patients. Results are expressed as percentage of control (untreated cells) as mean ± SD. Statistical analyses of the results were done by two-way ANOVA followed by Bonferroni post- test. *p<0.01, **p<0.005, ***p<0.001. N=3.
CXCR2 is overexpressed and modulates the expression of CXCL2 and CXCL8 in ACRP cells
Our previous data from cDNA microarray assays following NAC-1 KD in HGSOC cells [18] demonstrated that CXCL2 and CXCL8 are secreted in TME and can be correlated to chemoresistance (manuscript in preparation). We investigated this novel mechanism possibly underlying cisplatin resistance in EOC clinics. qRT-PCR experiments were run to evaluate CXCR2, CXCL2 and CXCL8 expression in ACRP cells. Our results revealed overexpression of CXCR2 in ACRP when compared to A2780 by 2.3-fold (p=0.034) (Figure 2A). Then, we downregulated the expression of CXCR2 in A2780 (p=0.0246) and in ACRP (p=0.001) by approximately 50% when compared to the negative control (NC) cells (Figure 2B). Further exploring the impact of CXCR2 expression in OC chemoresistance, we found that ACRP CXCR2 KD cells expressed 2.5-fold less CXCL2 (p=0.0362) (Figure 2C), and 6.5-fold less CXCL8 in comparison to NC cells (p=0.0025) (Figure 2D). Expression of CXCL2 and CXCL8 was not significantly modified in A2780 CXCR2 KD. Altogether, our results suggest the occurrence of an intricate CXCL2/CXCL8-CXCR2 axis in the modulation of cisplatin sensitivity by OC cells, thus corroborating with an important role of TME and a potential autocrine effect of CXCL2/CXCL8 on CXCR2 expressed by tumor cells.
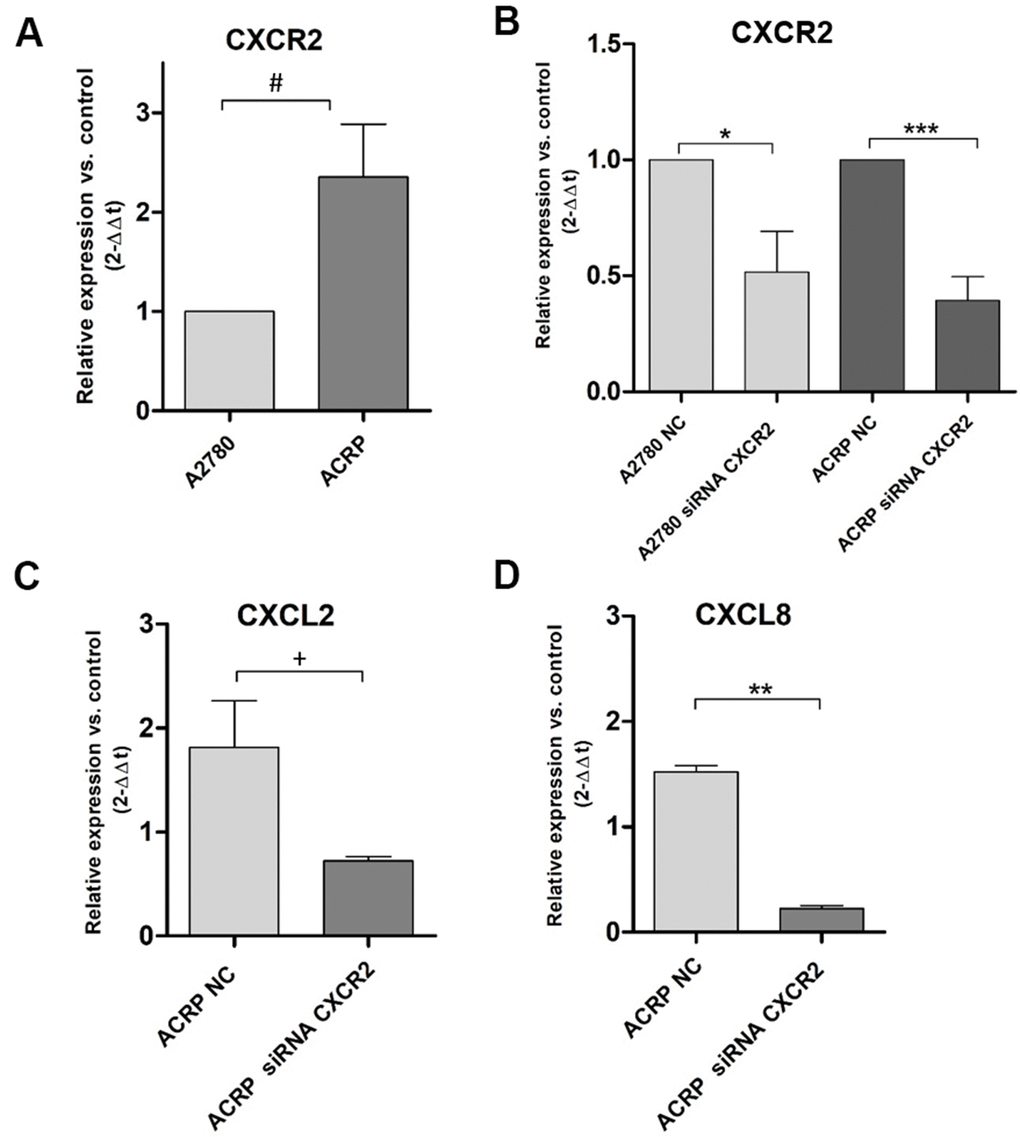
Figure 2. CXCR2 is overexpressed and modulates the expression of CXCL2 and CXCL8 in ACRP cells and CXCR2 KD. Transcript expression of CXCR2, CXCL2 and CXCL8 was investigated by qRT-PCR, following the protocol described in Material and Methods session, in both wild-type and CXCR2 KD (siRNA CXCR2, 10μM) A2780 and ACRP OC cells. (A) ACRP expressed 2.3-fold more CXCR2 than A2780 (#p=0.0342). (B) CXCR2 KD lead to lower expression of CXCR2 in both cell lines, however 1.3-fold less in ACRP (***p=0.001) than in A2780 (*0.0246). (C) CXCL2 expression was 2.5-fold lower in ACRP CXCR2 KD than in ACRP NC (+p=0.0362). (D) CXCL8 expression decreased by 6.5-fold comparing ACRP CXCR2 KD to ACRP NC (**p=0.0025). Differential gene expression was presented as relative expression of each gene of interest compared to control, after normalization by the expression of the housekeeping gene GAPDH and calculated by the 2-ΔΔCt method. Data were analyzed by unpaired t-Student test (p<0.05). N=3.
CXCR2 comprises nuclear expression in OC cells
Motivated by the aforementioned results, we decided to characterize the expression of CXCR2 in OC cells with regard to its cellular localization. To do so, we conducted immunofluorescence experiments with A2780 and ACRP, NC and CXCR2 KD (Figure 3, left column; nuclei stained with DAPI; blue). Treatment of cells with anti-CXCR2 antibody (red) revealed the overexpression of CXCR2 by ACRP in comparison with A2780 (middle column, Figure 3). CXCR2 was confined to the nuclei of A2780 and ACRP NC (Figure 3, right column). Nuclei expression of CXCR2 was sustained exclusively in ACRP CXCR2 KD (Figure 3, right column). Although the biological relevance of our observation remains unclear, it is imperative to point that chemoresistance to cisplatin is likely caused or aggravated by the sustained anomalous expression of CXCR2 in OC cells nuclei. In any event, we, herein, introduce a novel mechanism contributing to cisplatin chemoresistance in OC cells.
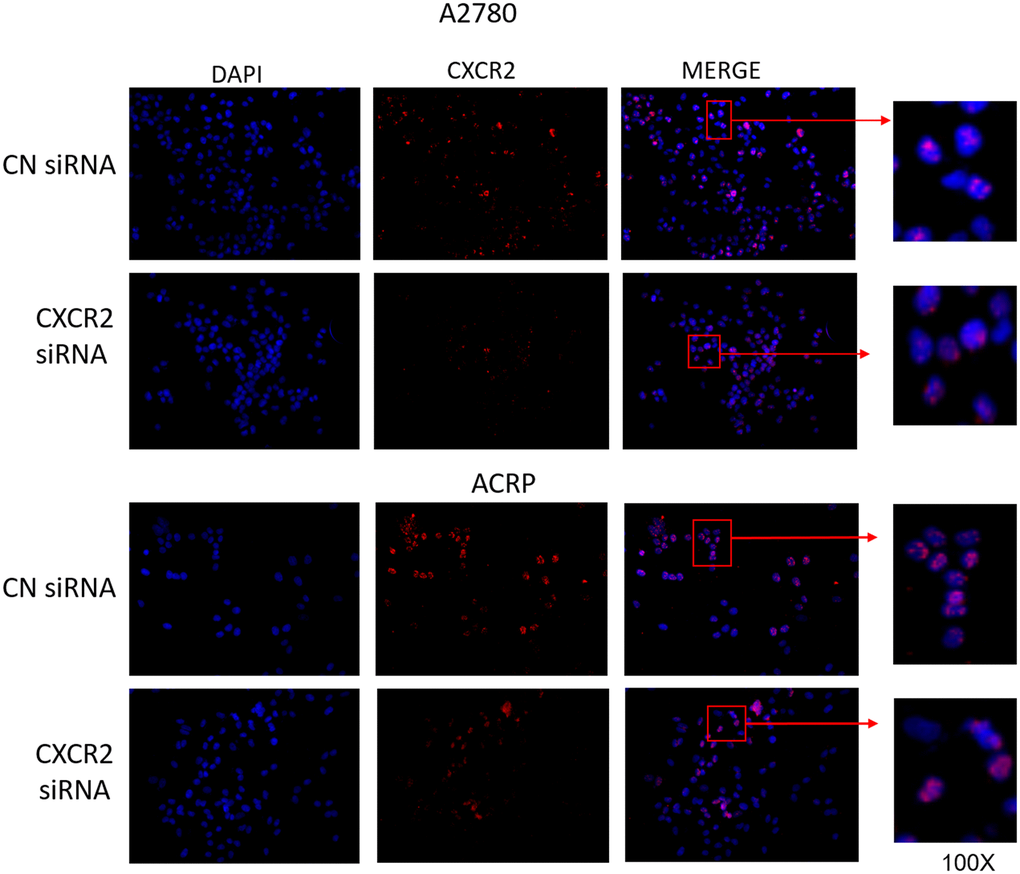
Figure 3. CXCR2 comprises persistent nuclei anomalous expression cisplatin resistant OC cells. Immunofluorescence assays were performed to investigate cellular localization of CXCR2 expression in OC cells. CXCR2 antibody (red) whereas cellular nuclei were stained with DAPI (blue); images where further merged to facilitate cellular localization analysis. Cells were plated at same density (left column of Figure). As previously demonstrated, CXCR2 was overexpressed in ACRP cells in comparison with A2780 cells (middle column of Figure). N=3. Images were acquired under 10x magnification.
CXCR2 overexpression correlates to patients’ low overall survival with primary EOC
Secretion of CXCL2 and CXCL8 in the conditioned medium of ACRP potentially act autocrinally on CXCR2 expressed by OC cells (manuscript in preparation). To evaluate the correlation between the expression of CXCR2 and EOC patients’ OS (overall survival), data from 370 patients diagnosed with primary EOC were obtained from TCGA database (Figure 4). Overexpression of CXCR2 was detected in 74.32% of the patients. EOC patients whose tumor cells expressed high levels of CXCR2 had lower OS compared with the cases expressing low levels of CXCR2 (n=0.035) (Figure 4A). No significant statistical differences were observed in OS of patients with EOC cells expressing low (n=109) or high (n=261) levels of CXCL2 (p=0.18) neither low (n=102) or high (n=268) levels of CXCL8 (p=0.95) (Figure 4B, 4C). Nonetheless, 70.54% and 72.43% of EOC cells overexpressed CXCL2 and CXCL8, respectively. Our results suggest that EOC cells can potentially secret CXCL2 and CXCL8 to TME that act autocrinally on tumor cells CXCR2, conferring the poor prognosis of the disease that is inferred by low patients’ OS. We postulate that CXCR2 emerges as a prognostic marker and a potential therapeutic target of EOC.
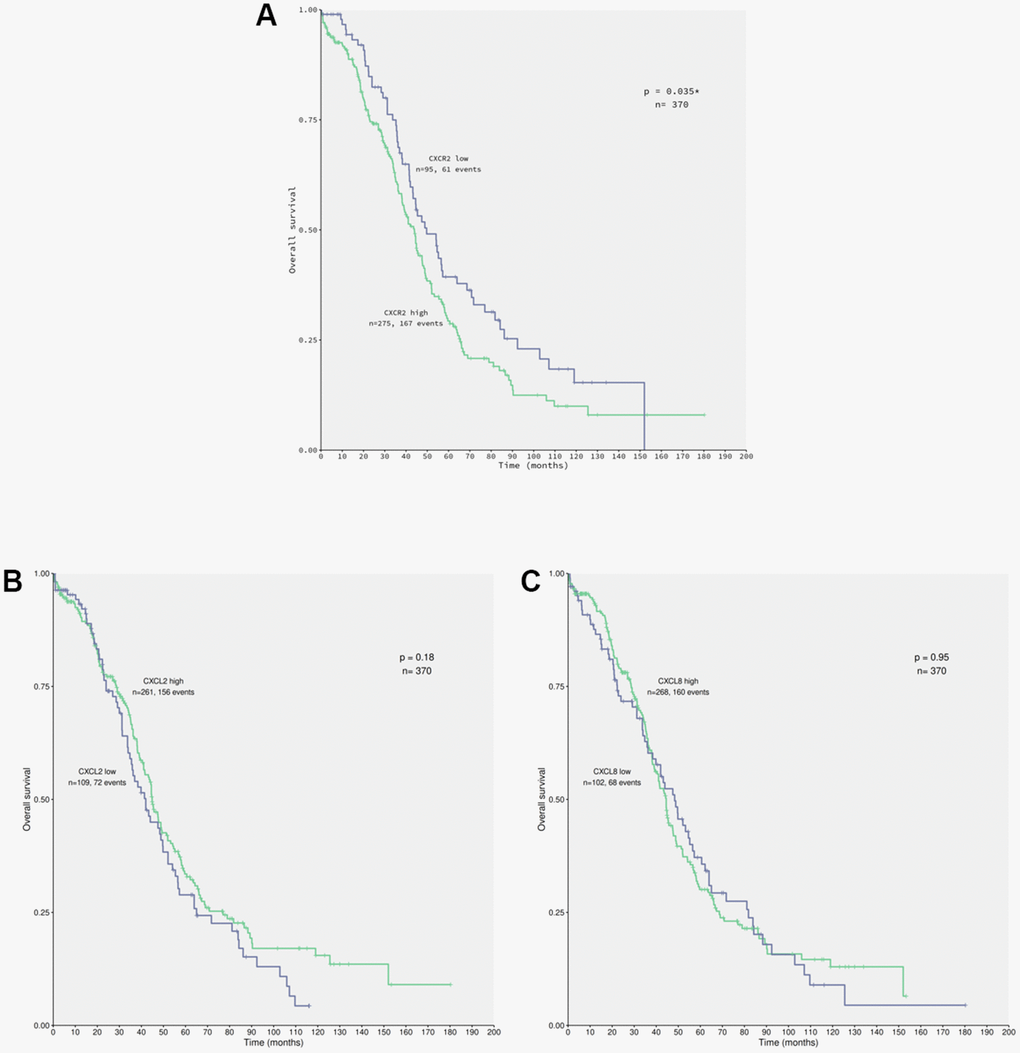
Figure 4. CXCR2 overexpression correlates to patients’ low overall survival carrying primary EOC. Data from 370 patients diagnosed with serous EOC were obtained from TCGA database for in silico analysis of patients’ overall survival (OS) in relation with the chemokines of interest expression by cancer cells, using the Kaplan-Meier statistical method. (A) Correlation of EOC patients, which tumor cells express low (n=95) or high (n=275) levels of CXCR2 to patients’ OS. Note that patients that carry EOC expressing high levels of CXCR2 had lower overall survival rate in comparison to the ones with low levels of the chemokines receptor (p=0.035). Moreover, overexpression of CXCR2 was identified in 74.32% of the studied patients. (B) Correlation of EOC patients, which tumor cells express low (n=109) or high (n=261) levels of CXCL2 to patients’ overall survival. No significant difference were observed with regard to CXCL2 expression by cancer cells and patients’ overall survival rate (p=0.18). However, overexpression of CXCL2 was seen in 70.54% of patients. (C) Correlation of EOC patients, which tumor cells express low (n=102) or high (n=268) levels of CXCL8 to patient’s overall survival. No significant differences were observed confronting CXCL8 expression by cancer cells to patients’ overall survival rate (p=0.95). Nonetheless, overexpression of CXCL8 was detected in 72.43% of patients. Long rank test was performed to analyse statistical difference amongst the parameters investigated.
CXCR2 enhances OC cell proliferation and cellular viability
To further explore the relevance of CXCR2 expression in OC cells with and without cisplatin resistance, we analysed proliferation and cellular viability in CXCR2 KD cells. BrdU assay revealed that in both A2780 and ACRP, CXCR2 KD led to a significant decrease in cell proliferation, of approximately 2.0-fold and 3.5-fold, respectively (Figure 5A–5C) (p<0.05). Treatment of cells with SB225002, which is a CXCR2 inhibitor, also resulted in decreased proliferation in both cell lines (about 50% reduction; p<0.05). Cell proliferation was lower in ACRP vs. A2780 in all events, thus supporting the role of CXCR2 in OC progression with regard to chemoresistance. Next, we performed clonogenic assays to evaluate OC cellular viability (Figure 6A, 6B) in CXCR2 KD cells. A2780 sensitivity vs. ACRP resistance to cisplatin was confirmed. ACRP CXCR2 KD were more sensitive than A2780 KD (p<0.001). There was a synergistic effect in loss of cell viability when CXCR2 KD cells were treated with cisplatin (A2780 p<0.001; ACRP p<0.01) or cisplatin and SB225002 (A2780 p<0.001; ACRP p<0.01), these data being more prominent in ACRP cells. These findings indicate that CXCR2 plays a central role in the acquisition of cisplatin chemoresistant phenotype by OC cells.
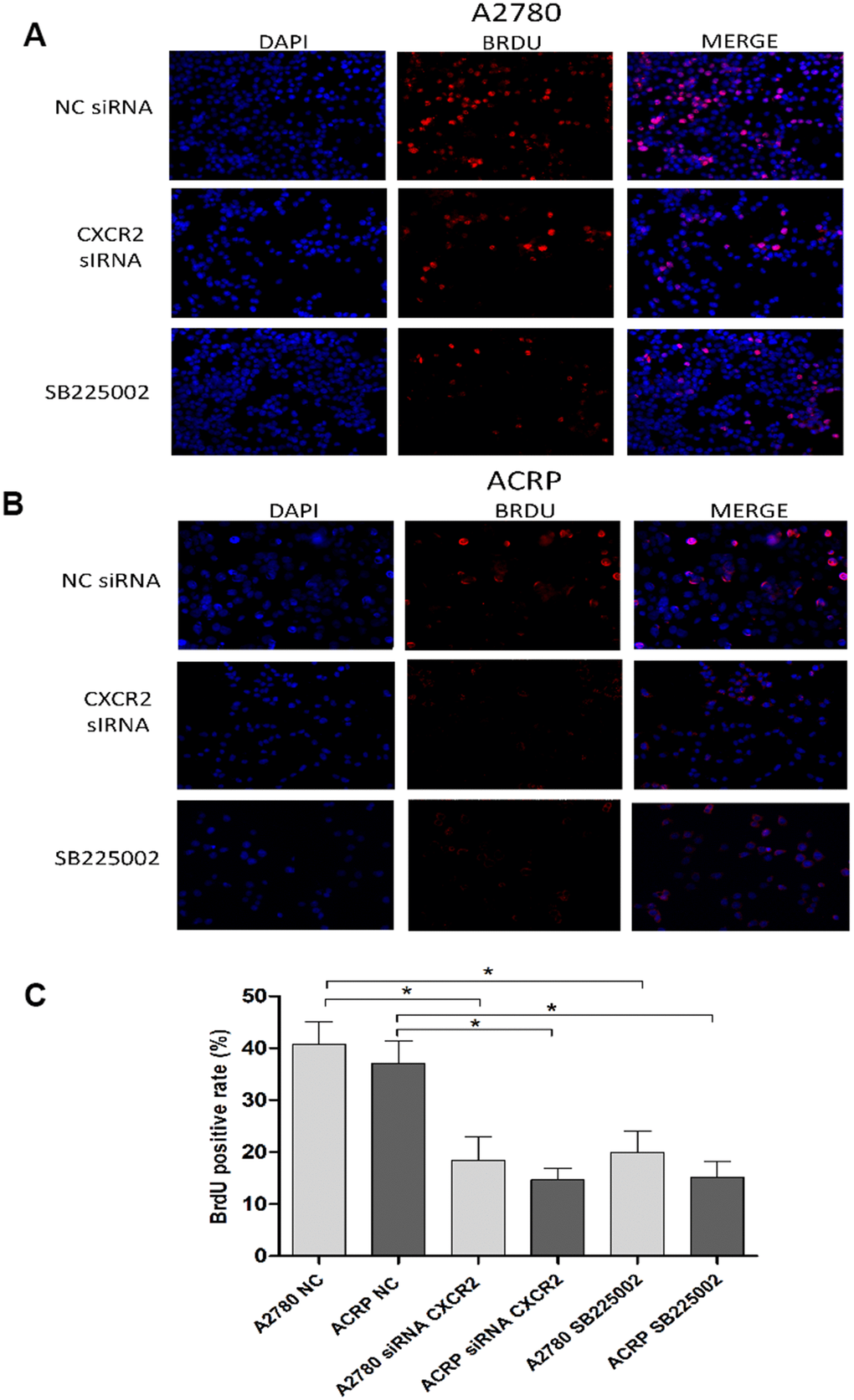
Figure 5. CXCR2 enhances OC cell proliferation. BrdU immunofluorescence assays were conducted to investigate the role of CXCR2 on OC cell proliferation. In brief, cells were plated at the same density and fixed, as previously reported. Antibody against the marker of cell proliferation, BrdU, was conjugated with PE (red), whereas cells nuclei were stained with DAPI (blue). Finally, both staining conditions were merged for better visualization of the studied phenomenon. (A) A2780 cells were either transfected with empty vector for control (NC), siRNA CXCR2 (10μM) or treated with the CXCR2 antagonist SB225002 (1ug/ml). (B) ACRP cells were either transfected with empty vector for control (NC), siRNA CXCR2 (10μM) or treated with the CXCR2 antagonist SB225002 (1ug/ml). (C) BrdU positive rate, indicating cell proliferation under each specified experimental condition. Note that cell proliferation decreased significantly both in the KD models and under treatment of cells with SB225002. Cell proliferation was remarkably lower in ACRP cells vs. A2780 cells in all events. Figure is of a representative experiment. Data was analyzed by one-way ANOVA. *p<0.05. N=3. Imagines were acquired in 10x magnification.
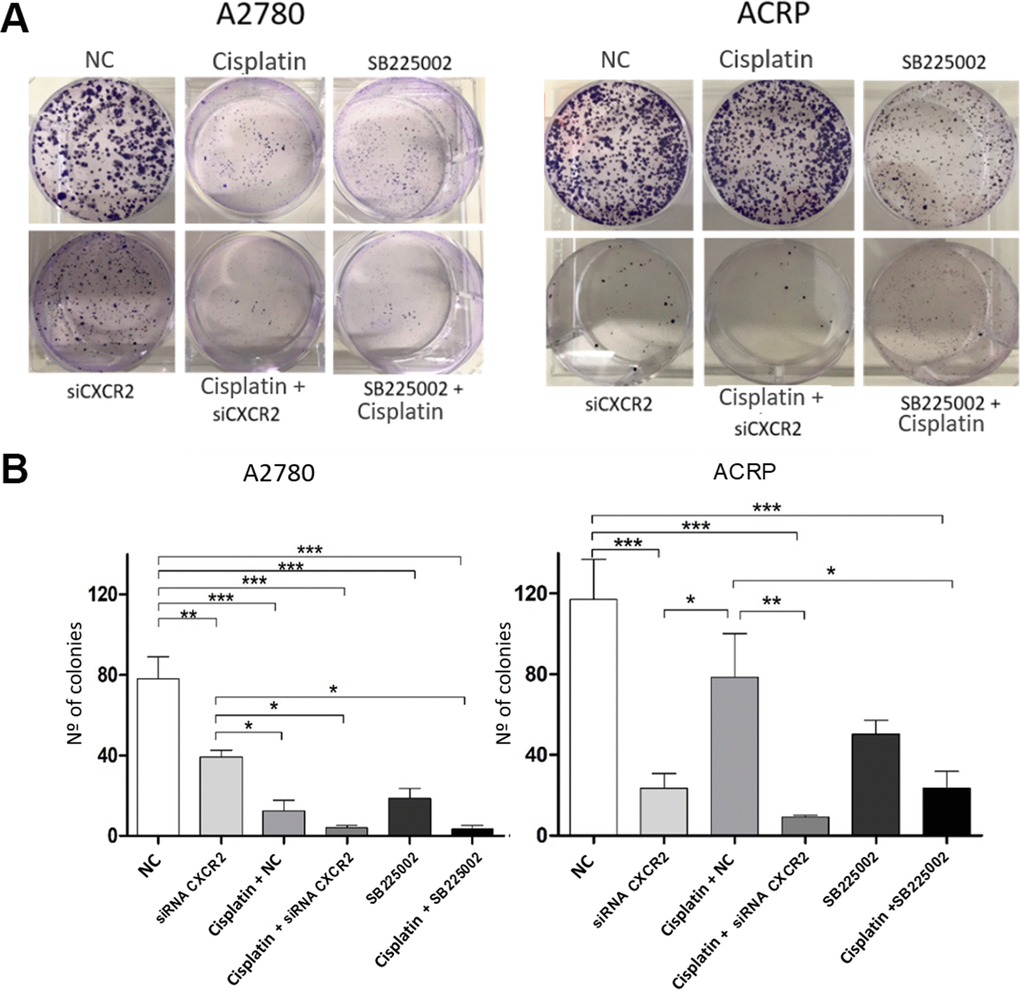
Figure 6. CXCR2 is a pivotal molecule in OC cellular viability. Clonogenic assays were run to evaluate the role of CXCR2 on OC cellular viability under the follow experimental conditions: i) cells transfected with empty vector (10μM), NC, control; ii) cells transfected with siRNA CXCR2 (10μM) to get the KD models; iii) cells that received cisplatin (5uM); iv) CXCR2 KD cells + cisplatin (5uM); v) cells treated with the CXCR2 antagonist SB225002 (1ug/ml); vi) cells that received combined therapy containing cisplatin (5uM) and SB225002 (1ug/ml). 150 cells of each lineage were plated on 6-well plates. Colonies were stained with crystal violet at D10. (A) Representative figure of the stained plate. The experiment confirmed A2780 sensitivity vs. ACRP resistance to cisplatin. A2780 CXCR2 KD cells were more viable than ACRP CXCR2 KD cells. Moreover, ACRP was more sensitive to SB225002 than A2780. On both cells, there was additive effects with the combined treatment containing cisplatin and siRNA against CXCR2 or cisplatin and SB225002. (B) Graphic representation of the percentage of colonies formed under each experimental condition, clearly reflecting Figure 5A. This experimental approach has proven the pivotal role of CXCR2 in the acquisition of cisplatin chemoresistant phenotype by OC cells. Data were analyzed by two-way ANOVA followed by Bonferroni post-test. *p<0.05, **p<0.01, ***p<0.001. N=3.
CXCR2 promotes in vivo OC tumor growth, angiogenesis and tumor invasion
Investigation of the role of CXCR2 on OC tumor growth (TG) and angiogenesis was assessed by the chicken embryo chorioallantoic membrane (CAM) method. Tumor size was measured at E10 egg inoculated with A2780 or ACRP, NC and CXCR2 KD. When compared to A2780, TG was higher in ACRP. Moreover, CXCR2 KD prevented TG in ACRP (Figure 7A, 7B). There were no differences amongst cells with regard to angiogenesis and invasion (Figure 7A–7C). Statistic significant differences were not observed probably due to the number of replicates performed. However, our results point to a potential biological importance of CXCR2 in cisplatin resistant OC cells, inferred by continuous TG, which is partially, but significantly, reversed by CXCR2 KD.
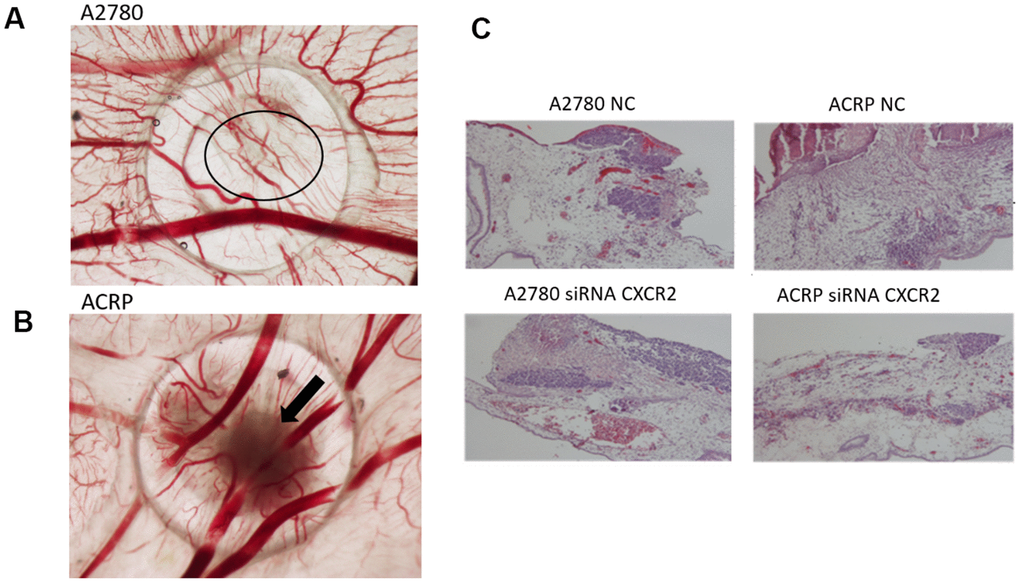
Figure 7. CXCR2 promotes in vivo OC tumor growth (TG), angiogenesis and tumor invasion.In vivo investigation of the role of CXCR2 on tumor growth, invasion and angiogenesis in OC was assessed by chicken embryo chorioallantoic membrane (CAM) method. (A) The number of new vessels formation with diameter lower than 20μm growing radially towards the ring area was counted in a blind fashion manner. No differences were noted between the lineages under the referred experimental condition. (B) Tumor size was measured at E10 egg inoculation with A2780 and ACRP OC cells, as: i) NC; ii) siRNA CXCR2 KD cells. Statistic significant difference were not observed probably due to number of replicates performed. However, when compared to A2780, TG was higher in ACRP than in A2780 cells. Moreover, CXCR2 KD prevented TG in later. The overall mortality rate for embryos/eggs was as expected (~10%). (C) The figure is also of a distinct invasion patterns between NC and CXCR2 KD ACRP and A2780 cells.
Silencing CXCR2 expression reduces EMT marker proteins SLUG and SNAIL in ACRP and seems to modulate PI3K/AKT/mTOR, but not MEK/ERK, pathway
Studies published so far have associated CXCR2 expression in cancer cells to the epithelial-mesenchymal transition (EMT) phenotype [25, 26]. Thus, we questioned whether this could also contribute to CXCR2-induced chemoresistance to cisplatin in OC cells. We analyzed protein expression of EMT markers as Snail, Slug and β-Catenin, and noted an increase in the expression of Snail and Slug in ACRP, which is reversed by CXCR2 KD (Figure 8B–8D). These data suggest that EMT is likely involved in CXCR2-dependent cisplatin chemoresistance in OC cells. These results were not observed when cells were treated with SB225002. In contrast, inhibition of CXCR2 did not significantly modulate vimentin nor reversed β-catenin expression in our study model (Figure 8D, 8E). We hypothesize that there might be a CXCR2-SNAIL-SLUG axis contributing to the CXCR2 role in cisplatin chemoresistance in OC cells. We, then, argued if silencing CXCR2 expression could modulate the classical carcinogenic signaling pathways PI3K/AKT/mTOR and MEK/ERK, leading to an observation of a biological tendency to decrease the expression of p-AKT (0.5-fold) in ACRP CXCR2 KD but not in A2780 KD (p=0.07 and p=0.09, respectively) (Figure 8F, 8G).
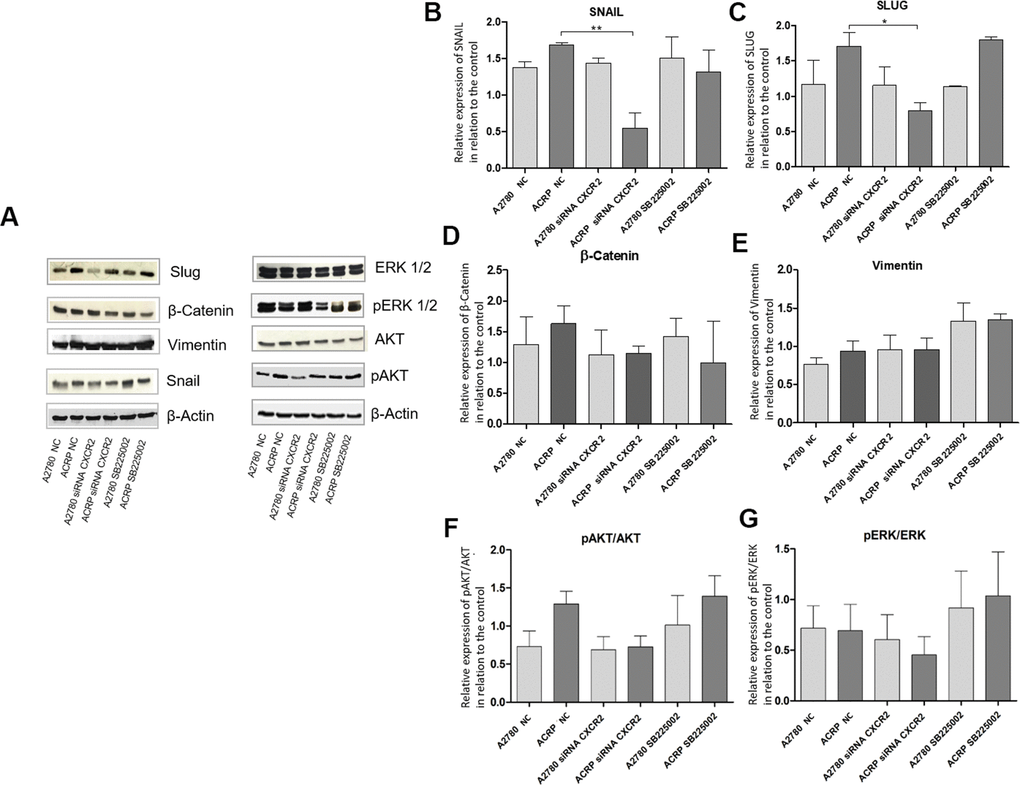
Figure 8. Silencing CXCR2 expression reduces EMT marker proteins SLUG and SNAIL in ACRP and seems to modulate PI3K/AKT/mTOR, but not MEK/ERK, pathway. Western blot assays were performed to investigate the expression of EMT marker proteins in ACRP and A2780 lines, as follow: i) CN cells (10; II) siRNA CXCR2 KD cells; iii) cells treated with SB225002. 50μ of protein were loaded into SDS-PAGE gels, proteins were separated by electrophoresis and blotted with the primary antibodies of interest. (A) Representative figure of the blots performed for each marker and different treatments, (B) SNAIL protein expression was significantly decreased in ACRP CXCR2 KD cells vs. ACRP wild type, but not in A2780 cells. (C) SLUG protein expression was significantly lower in ACRP CXCR2 KD cells with comparison to its wild-type counterpart, however not in A2780 cells. (D) β-Catenin and (E) Vimentin did not present significant statistical difference amongst the conditions studied. Moreover, when we seek to correlate pro-carcinogenic signaling pathways related to CXCR2, no statistic significant difference were noted both in (F) pAKT/AKT and (G) pERK/ERK pathways. It is worth note to address the biologic tendency of the CXCR2 KD models, but not the treatment of cells with SB225002, to inactivate PI3K/AKT/mTOR, but not MEK/ERK pathway (p= 0.7 and p=0.09 in ACRP and A2780, respectively). Data were analysed by two-way ANOVA followed the Bonferroni post- test. β-actin was used as a normalization control of the experiments. *p<0.022, **p<0.005. N=3.
Discussion
In this study, we created an OC in vitro model of pan-resistant cells to cisplatin, paclitaxel and doxorubicin, thus reproducing a major challenge in fighting OC, which is recurrence of aggressive and lethal disease due to cisplatin chemoresistance. We further demonstrated that resistant cells depend on CXCR2 for survival and aggressiveness, at least partially through an EMT phenotype.
We aimed to elucidate the contribution of TME on chemoresistance motivated by our previous observations that ACRP cells secret CXCL2 and CXCL8 to their conditioned medium that ultimately modulate the fate of OC (manuscript in preparation). The present results suggest an autocrine effect of CXCL2/CXCL8 through CXCR2 expressed by OC cells. CXCL2 and CXCL8 have been correlated to cancer cells chemoresistance, migration, angiogenesis and progression in melanoma, colon and ovary cancers [19, 20, 27–29]. As previously mentioned, CXCL2 and CXCL8 depend on their specific binding to CXCR2 to elicit their cellular functions, a membrane receptor that has been detected in endothelial cells, infiltrating neutrophils, and tumor-associated macrophages, which also suggests an important regulatory role within the TME [30].
ACRP overexpresses CXCR2 in comparison to A2780. CXCR2 KD models were confirmed by the decrease of CXCR2 expression in both lineages when compared NC cells, being the effect more prominent in ACRP CXCR2 KD than in A2780 CXCR2 KD. The expression of CXCL2 and CXCL8 was significantly decreased in ACRP CXCR2 KD when compared to ACRP NC, but similar results were not observed in A2780. These results point to the occurrence of an intricate CXCR2/CXCL2-CXCL8 axis possibly modulating cisplatin resistance in OC and thus supporting the hypothesis of a role of the TME and a potential autocrine effect of chemokines on CXCR2 expressed by tumor cells.
Motivated by our results, we then questioned whether the cellular localization of CXCR2 could be altered in OC cells. Surprisingly, CXCR2 expression, which was significantly higher in ACRP than in A2780, was restricted to cellular nuclei instead of trafficking to the OC cells membrane. Although further studies are needed to elucidate the biological function of our findings, it is, at least to our knowledge, a novel observation. Nonetheless, recent data from the literatures cannot be neglected. DEK, an endogenous chromatin-binding factor that regulates hematopoiesis, can bind to CXCR2 and sequestrate it to the cellular nucleus. We hypothesized that binding of DEK to CXCR2 may, at least partially, justify the persistent nuclear expression of CXCR2 in the nuclei of OC cells, as DEK exerts its function in cellular nuclei in a CXCR2-dependent manner [31].
Overexpression of CXCR2 was previously associated with early recurrence of OC [24, 32]. In this study, information from 370 patients diagnosed with primary EOC was obtained from the TCGA database. Patients’ OS was opposed to CXCR2 overexpression in EOC cells, clearly demonstrating that CXCR2 is a marker of the disease poor prognosis. Similar experiments were run in intrahepatic, lung, cholangiocellular, pancreas, kidney, breast and colon cancer samples, also correlating poor disease prognosis to CXCR2 overexpression by cancer cells [22, 33–35].
Cell proliferation was lower in ACRP CXCR2 KD and in A2780 CXCR2 KD rather than when they were treated with SB225002, thus corroborating with the differential effects of CXCR2 KD and SB225002 treatment in OC cells and supporting the need to develop CXCR2 inhibitors that permeate cellular membrane to reach its target in cellular nuclei. The effect of CXCR2 on cell proliferation was shown in melanoma [36, 37] ovary [24] and prostate cancers [38, 39]. We found that A2780 was more sensitive to cisplatin than ACRP CXCR2 KD. Cellular viability of ACRP CXCR2 KD was lower than that of A2780. Our data show the greater effect of gene silencing than pharmacological intervention in ACRP, which suggest that resistant cells likely present intracellular expression of CXCR2, as proven in this work. Furthermore, cell viability decreased when CXCR2 was inhibited by SB225002 or CXCR2 KD in monotherapy. Moreover, there was synergy of these treatment approaches with cisplatin in both cell lines, being the effect more eminent in ACRP than A2780. A study conducted in OC demonstrated the synergic effect of the combined therapy containing sorafenib and SB225002 in inhibiting cell proliferation and angiogenesis [40]. In HGSOC cells SKOV3, CXCR2 KD comprised secretive activity of CXCL1 and CXCL8 [24] and, likewise our results, led to decreased cell viability, suggesting that silencing CXCR2 expression suppressed OC tumorigenicity in vivo and in vitro. Metastatic breast cancer cells can be re-sensitized to paclitaxel and doxorubicin by CXCR2 KD [41], as CXCR2 blockade was correlated to increased overall therapeutic response to antineoplastic substances, possibly due to lower TG and metastasis indexes [33, 41]. Significant inhibition of progression of CXCR2-negative metastatic lung cancer treated with paclitaxel was observed [33]. These findings are in agreement with ours through which CXCR2 emerge as a novel molecule orchestrating sensitivity of cancer cells to chemotherapy. Furthermore, CXCR2 arises as an unprecedented target to fight cancer in the adjuvant setting by overcoming chemotherapy resistance [33].
CXCR2-induced cell proliferation seems mediated by PI3K/AKT/mTOR and MEK/ERK pathways, which modulate cell cycle, apoptosis and angiogenesis, as well as the secretion of chemokines (as CXCL8) and cytokines (as seen for IL6) [Reviewed in 42]. The chemokine-regulated pathways led to cell adhesion, migration, chemotaxis, changes in cell morphology and regulation or activation of integrin [43, 44]. In our study model, though we did not find significant difference, one might suggest a biological tendency of CXCR2 to activate PI3K/AKT/mTOR, but not MEK/ERK pathway in OC cells.
Another important aspect to address is whether CXCR2 affects TG, angiogenesis and invasion of OC cells. CXCR2 stimulated TG, invasion and metastasis in murine KRAS/p53-mutant lung adenocarcinoma cell line [22]. In contrast, CXCR2 seems to induce TG in an angiogenesis-independent fashion in ACRP, however not in A2780. Further experiments are necessary to confirm this hypothesis.
EMT has been implicated as a key process involved in tumor invasion and metastasis, affecting characteristics such as stemness, apoptosis and immune system [45]. Snail, an EMT marker, promoted CXCR2 ligand-dependent tumor progression in lung cancer [46]. Moreover, EMT was directly related to chemoresistance [47–49]. Our results showed overexpression of EMT markers, such as Snail and Slug, in ACRP vs. A2780. We reported that ACRP overexpressed Snail, Slug and β-catenin. Nevertheless, only overexpression of Snail and Slug were reversed in ACRP CXCR2 KD. Snail is a transcriptional repressor of E-cadherin and induces trafficking of myeloid-derived suppressor cells via upregulation of CXCR2. In silico analysis of EOC data obtained from TCGA database indicated that Snail is correlated with the secretion of CXCL2/CXCL8 to TME [49, 50], thus revealing that Snail has multiple important functions, including modulation of the immune system and EMT.
In conclusion, our study proves that CXCR2 is retained in the nucleus of OC cells that acquired cisplatin resistant phenotype, being correlated with poor prognosis of the disease and its high mortality rate in patients. In addition, CXCR2 is associated with tumor proliferation and growth in OC resistant cells, showing its role in disease chemoresistant phenotype acquisition and progression. Thus, effective strategies through the synthesis of highly lipophilic analogous molecules of the prototype CXCR2 competitive inhibitor SB225002 can inhibit CXCR2 carcinogenic role in OC, in an economical and clinical viable fashion. In any event, we, herein, introduce a novel mechanism that contributes to chemoresistance of OC cells to cisplatin.
Our study has proven that the inhibition of CXCR2 pathway may not only lead to OC antitumor properties but may also act as a chemosensitizer of tumor cells to cisplatin. In summary, our results present innovative strategy to treat pan-chemoresistant OC, by inhibiting the persistent and anomalous nuclear overexpression of CXCR2 in cisplatin resistant disease, therefore, opening a novel avenue to combat this still highly deadly disease.
Materials and Methods
Cell lines and culture conditions
Pan-resistant ACRP cells were generated from its parental counterpart A2780 lineage, following chronic exposure to cisplatin (1μM to 10 μM). Chemoresistance was verified by the MTT method [51] through the calculation of estimated IC50 of cisplatin, paclitaxel and doxorubicin. Lineages were cultured in complete DMEM medium (Invitrogen) supplemented with FBS 10%(v/v), penicillin/streptomycin1%(w/v), amphotericin1%(w/v), at 37° C in atmosphere of 5% CO2.
Generation of CXCR2 KD cells
CXCR2 gene expression was silenced using small interfering siRNACXCR2 plasmid (10 μM), 5μl of lipofectamine 2000 in 125μl of Opti-MEM® Reduced-Serum Medium, as manufacturer suggestion (Invitrogen). Control experiments were run in parallel, using Stealth RNAi siRNA Negative Control Duplex (10μM) (Invitrogen). CXCR2 was also pharmacologically inhibited by SB225002 (1ug/mL) (Abcam) diluted in 0.001%(v/v) dimethylsulfoxide (DMSO).
RNA extraction and real-time reverse transcription polymerase chain reaction (qRT-PCR)
RNA was extracted using TRIzol (Invitrogen), following the manufacturer instructions. cDNA was obtained by SuperScript First-Strand Synthesis System (Invitrogen; manufacturer protocol). For quantitative q-RT-PCR reactions, 50ng cDNA were amplified in SYBR Green PCR Master Mix (Applied Biosystems). Gene expression was annotated as 2-ΔΔC, using ABI Prism 7500 Fast System software (Applied Biosystems). Amplification conditions were 95° C for DNA denaturation, melting temperature 58° C and 72° C for DNA extension; 40 cycles. Primers were: CXCR2: F3’TTGCAACCCAGGTCAGAAGTT5’ (10μm), R3’CAGCTGTGACCTGCTGTTATT5’ (10μm);
GAPDH: F3’CAGCCTCAAGATCATCAGCA5’ (10μm), R3’ACAGTCTTCTGGGTGGCAGT5’ (10μm) (Invitrogen).
Clonogenic assay
CXCR-modulated cell viability was investigated in CXCR2 KD cells or lineages treated SB225002 (1μg/mL) (150 cells/well). Cells were harvested at D10 cisplatin treatment (5μM). Colony formation was analysed by crystal violet staining [52]. Experimental controls were done using empty siRNA plasmids or wells containing only DMSO 0.001%(v/v).
Western blot
EMT markers, PI3K/AKT/mTOR and MEK/ERK signalling pathways elements were analysed by Western blot. Total proteins were extracted from cells using RIPA buffer (NaCl 1%w/v, sodium deoxycholate 0.5%w/v, SDS 0.1%w/v), Tris (50mM; pH 8.00). 30μg of protein were applied to 10%(w/v) polyacrylamide gels, separated by SDS-PAGE electrophoresis, then transferred to PVDF membranes (Millipore, USA). Membranes were blocked with skim milk 5%(w/v) or BSA 5%(w/v) for 30 minutes, then incubated overnight with primary antibody (1:1000) at 4° C. Anti-β-actin (1:4000) was used as internal control for semi-quantitative analysis. After incubation with secondary antibody (60 minutes), at room temperature (RT), blots were revealed with ECL reagent (manufacturer protocol) (GE). Protein expression was analysed by Lab software 6.1 version for Windows (Bio Rad).
Immunofluorescence
Cells were fixed on glass slides with methanol 20%(v/v), embedded in HCL 2M for 30 minutes at RT, incubated with anti-BrdU (1:10) or anti-CXCR2 (1:200) (Abcam) for 60 minutes at RT, then incubated with Alexa Fluor 494 goat anti-mouse or Alexa Fluor 494 goat anti-rabbit secondary antibodies (1:100) (Invitrogen) for 60 minutes, at RT, in the dark. Cells nuclei were stained with DAPI (1:10) (Thermo Fisher) for 15 minutes at RT. Images were acquired in Zeiss Z1 apotome microscope (LEICA) at 10x magnification.
Chicken embryo chorioallantoic membrane (CAM) angiogenesis and tumor growth assay
Chicken embryo chorioallantoic membrane (CAM) method was used to evaluate angiogenesis and tumor growth (TG) [53, 54]. Fertilized Gallus gallus eggs were incubated horizontally at 37.8° C in a humidified atmosphere (embryonic day; E). On E3, a square window was opened on the shell after removal of 2-2.5mL of albumen. Window was sealed and eggs were returned to incubator. At E10, 106 cells were placed into a 3 mm silicon ring, under sterile conditions, on top of growing CAM. Eggs were re-sealed and returned to incubator for 4 days. After removing the ring, CAM was excised from embryos, photographed under stereoscope at 20x magnification (Olympus, SZX16 coupled with DP71 camera). The number of new vessels (less than 20 μm diameter) growing radially towards the ring area was counted in a blind fashion manner, as well as the observation of tumor growth and invasion.
Survival analysis
In silico analysis was performed to correlate EOC patients’ overall survival rate (OS) with CXCR2 expression from 370 patients diagnosed with primary EOC were extract from The Cancer Genome Atlas Program (TCGA Computational Tools) [55].
Statistical analysis
Results are presented as mean ± SD. Statistical significance was calculated by unpaired t-Student test, one-way ANOVA or two-way ANOVA followed Bonferroni post-hoc test, as indicated in Figure legends (GraphPad Prism software version 5.00 for Windows). In silico patients’ OS analysis was expressed as Kaplan-Meier curves. p<0.05 was considered for statistical significance.
EOC: Epithelial Ovarian Cancer;
HGSOC: High Grade Serous Ovarian Cancer;
KD: Knockdown;
EMT: Epithelial-mesenchymal transition;
NC: Negative Control;
OC: Ovarian cancer;
TME: tumor microenvironment;
OS: Overall Survival;
TG: Tumor growth;
CAM: Chicken embryo chorioallantoic membrane.
The authors declare that they have no conflicts of interest.
CAPES, CNPQ and FAPES.