Introduction
Aging
of multicellular and unicellular eukaryotic organisms is a multifactorial
biological phenomenon that has various causes and affects a plethora of
cellular activities [1]. These numerous activities are modulated by only a few
nutrient- and energy-sensing signaling
pathways
that are conserved across phyla and include the insulin/insulin-like growth
factor 1 (IGF-1), AMP-activated protein kinase/target
of rapamycin (AMPK/ TOR)
and cAMP/protein kinase A (cAMP/PKA) pathways [2-5]. By sharing a compendium of
protein kinases and adaptor proteins, the insulin/IGF-1, AMPK/TOR and cAMP/PKA
pathways in yeast, worms, fruit flies and mammals converge into a network
regulating longevity [2-4],6,7]. This network may also include several proteins
that currently are not viewed as being in any of these three pathways
[2,3,8,9]. Moreover, this network responds to the age-related partial
mitochondrial dysfunction and is modulated by mitochondrially produced reactive
oxygen species (ROS) [3,8,10,11]. By sensing the nutritional status of the
whole organism as well as the intracellular nutrient and energy status,
functional state of mitochondria, and concentration of ROS produced in
mitochondria, the longevity network regulates life span across species by
coordinating information flow along its convergent, divergent and multiply
branched signaling pathways.
By
defining the organismal and intracellular nutrient and energy status, nutrient
intake plays an important role in modulating life span and influences
age-related pathologies [12,13]. Two dietary regimens are known to have the
most profound life-extending effects across species and to improve overall
health by delaying the onset of age-related diseases. They include: 1) caloric
restriction (CR), a diet in which only calorie intake is reduced but the supply
of amino acids, vitamins and other nutrients is not compromised [13-15]; and 2)
dietary restriction (DR), in which the intake of nutrients (but not necessarily
of calories) is reduced by limiting food supply without causing malnutrition
[16-18]. In a "TOR-centric" view of longevity regulation, TOR alone governs the
life-extending and health-improving effects of CR/DR by: 1) integrating the
flow of information on the organismal and intracellular nutrient and energy
status from the protein kinases AMPK, PKA, PKB/AKT (the insulin/IGF-1 pathway)
and ERK1/2 (the PKA-inhibited Raf/MEK/ERK protein kinase cascade) as well as
from the mitochondrial redox protein P66Shc; 2) sensing the
intracellular levels of amino acids in an AMPK-independent manner; and 3)
operating as a control center which, based on the information it has gathered
and processed, modulates many longevity-related processes in a
sirtuin-independent fashion [19-21]. The inability of CR to increase the
replicative life span (RLS) of yeast mutants lacking components of the TOR
pathway [22] and the lack of the beneficial effect of DR on life span in worms
with reduced TOR signaling [23,24] support the proposed central role for TOR in
orchestrating the life-extending effect of CR/DR in these two longevity
paradigms. Moreover, while the postulated by the
TOR-centric model dispensability of sirtuins for the longevity benefit
associated with DR has been confirmed in worms [24], the importance of the
sirtuin Sir2p in mediating the life-extending effect of CR in replicatively
aging yeast is debated [22,25-[27]. Noteworthy, while TOR is a central
regulator of the life-extending effect of CR in replicatively
aging yeast, the longevity benefit associated with CR in chronologically
aging yeast is mediated by a signaling network that includes: 1) the TOR and cAMP/PKA pathways converged on Rim15p, which
therefore acts as a nutritional integrator; and 2) some other, currently
unknown pathways that are not centered on Rim15p [6]. Considering the likely
convergence of the insulin/IGF-1, AMPK/TOR and cAMP/PKA signaling pathways into
a network regulating longevity in worms, fruit flies and mammals (see above),
it is conceivable that - akin to TOR - the insulin/IGF-1 and cAMP/PKA pathways
may contribute to the beneficial effect of CR/DR on their longevity. Although
some findings in worms, fruit flies and mammals support the involvement of the
insulin/IGF-1 pathway in the longevity benefit associated with CR/DR, other
data imply that such benefit is independent of insulin/IGF-1 (reviewed by
Narasimhan et al. [3]). The role of cAMP/PKA
signaling in the life-extending effect of CR/DR in these multicellular
eukaryotes remains to be tested. Importantly, the recently reported in worms
involvement of both independent and overlapping pathways in life span extension
by different DR regimens [28] supports the notion that the longevity benefit
associated with nutrient limitation is mediated by a signaling network that
integrates several pathways.
Akin to CR and DR regimens, certain
pharmacological interventions can extend longevity across phyla and improve
health by beneficially influencing age-related pathologies. Noteworthy, all of
the currently known anti-aging compounds increase life span under non-CR or
non-DR conditions (Supplementary Table 1). Under such conditions, these compounds have been
shown to: 1) provide the longevity and health benefits associated with CR and
DR, but without restricting caloric and nutrient intake; and 2) mimic numerous
life-extending effects of CR and DR on gene expression pattern, metabolic and
physiological processes, and stress response pathways. Therefore, the names "CR
mimetics" and "DR mimetics" have been coined for them [29,30]. Importantly,
most CR mimetics and DR mimetics target signaling pathways that modulate
longevity in response to the organismal and intracellular nutrient and energy
status, including the insulin/IGF-1 and AMPK/TOR pathways as well as the sirtuin-governed
protein deacetylation module of the longevity signaling network integrating
these pathways (Supplementary Table 1). Furthermore, such compounds as resveratrol,
metformin and mianserin increase life span only under non-CR or non-DR
conditions, but are unable to do so if the supply of calories or nutrients is
limited [31-35]. Hence, one could envision that most, if not all, longevity
pathways are "adaptable" by nature, i.e., that they modulate longevity
only in response to certain changes in the extracellular and intracellular
nutrient and energy status of an organism. However, Li+ in worms and
rapamycin in fruits flies extend life span even under DR conditions [36,37]. It
is likely therefore that some longevity pathways could be "constitutive" or
"housekeeping" by nature, i.e., that they: 1) modulate longevity
irrespective of the organismal and intra-cellular nutrient and energy status;
and 2) do not overlap (or only partially overlap) with the adaptable longevity
pathways that are under the stringent control of calorie and/or nutrient
availability. The challenge is to identify such housekeeping longevity
pathways, perhaps by using a chemical screen for compounds that can extend
longevity even under CR/DR conditions. Because under such conditions the
adaptable pro-aging pathways are fully suppressed and the adaptable anti-aging
pathways are fully activated, a compound that can increase life span is
expected to target the housekeeping longevity pathways.
Noteworthy,
two anti-aging compounds alter lipid levels in mammals and fruit flies under
non-DR conditions. In fact, resveratrol treatment reduces the levels of the
neutral lipids triacylglycerols (TAG) and increases free fatty acid (FFA)
levels in mouse adipocytes [38]. Furthermore, feeding rapamycin to fruit flies
results in elevated TAG levels [37]. Although it remains to be seen if such
effects of resveratrol and rapamycin on lipid levels play a casual role in
their anti-aging action under non-DR conditions, it should be stressed that
lipid metabolism has been shown to be involved in longevity regulation in yeast
[39,40], worms [9,41-43],
fruit flies [41,44] and mice [38,41,45-48]. We
recently proposed a mechanism linking yeast longevity and lipid dynamics in the
endoplasmic reticulum (ER), lipid bodies and peroxisomes. In this mechanism, a
CR diet extends yeast chronological life span (CLS) by activating FFA oxidation
in peroxisomes [39-40]. It is conceivable that the identification of small
molecules targeting this mechanism could yield novel anti-aging compounds. Such
compounds can be used as research tools for
defining the roles for different longevity pathways in modulating lipid
metabolism and in integrating lipid dynamics with other longevity-related
processes. Furthermore, the availability of such compounds would enable a quest
for housekeeping longevity assurance pathways that do not overlap (or only
partially overlap) with the adaptable TOR and cAMP/PKA
pathways. Moreover, such compounds would have a potential to be used as
pharmaceutical agents for increasing life span and promoting healthy aging by
delaying the onset of age-related diseases, regardless of an organism's dietary
regimen.
We
sought to identify small molecules that increase the CLS of yeast under CR
conditions by targeting lipid metabolism and modulating housekeeping longevity
assurance pathways. Our chemical genetic screen identified lithocholic acid
(LCA) as one of such small molecules. We provide evidence that LCA extends
longevity of chronologically aging yeast through two different mechanisms. In
one mechanism, this bile acid targets - regardless of the number of available
calories - housekeeping longevity assurance pathways that do not overlap with
the adaptable TOR and cAMP/PKA pathways and modulate a compendium of pro- and
anti-aging processes. In the other mechanism, LCA targets the adaptable
cAMP/PKA pathway under non-CR conditions by unmasking the previously unknown
anti-aging potential of PKA.
Results
Our
rationale for choosing a mutant strain and growth conditions to screen compound
libraries for anti-aging small molecules
To
perform a chemical genetic screen for small
molecules that increase the CLS of
yeast by targeting lipid metabolism, we chose the single-gene-deletion
mutant strain pex5Δ.
Because pex5Δ lacks a
cytosolic shuttling receptor for peroxisomal import of Fox1p and Fox2p, these
two enzymes of the β-oxidation of FFA reside in the cytosol of pex5Δ cells [49] (Figure 1A). In
contrast, the Pex5p-independent peroxisomal import of Fox3p, the third enzyme
of the FFA β-oxidation pathway, sorts it to the peroxisome in pex5Δ cells [49]. By spatially
separating Fox1p and Fox2p from Fox3p within a cell, the pex5Δ mutation impairs FFA oxidation
(Figure 1A). In chronologically aging yeast
grown under CR conditions on 0.2% or 0.5% glucose, peroxisomal FFA oxidation
regulates longevity by 1) efficiently generating acetyl-CoA to synthesize the
bulk of ATP in mitochondria; and 2) acting as a rheostat that modulates
the age-related dynamics of FFA and diacylglycerol (DAG), two regulatory lipids
known to promote longevity-defining cell death [39,40,50]. Unlike CR yeast, chronologically aging non-CR yeast grown on 1% or
2% glucose are unable to generate significant quantities of ATP by oxidizing
peroxisome-derived acetyl-CoA in mitochondria and, instead, produce the bulk of
ATP via glycolytic oxidation of glycogen- and trehalose-derived glucose
[39,40]. Consistent with the essential role of peroxisomal FFA oxidation as a
longevity assurance process only under CR, the pex5Δ mutation substantially shortened
the CLS of CR yeast but caused a significantly lower reduction of longevity in
non-CR yeast, especially in yeast grown on 2% glucose (Figures 1B to F).
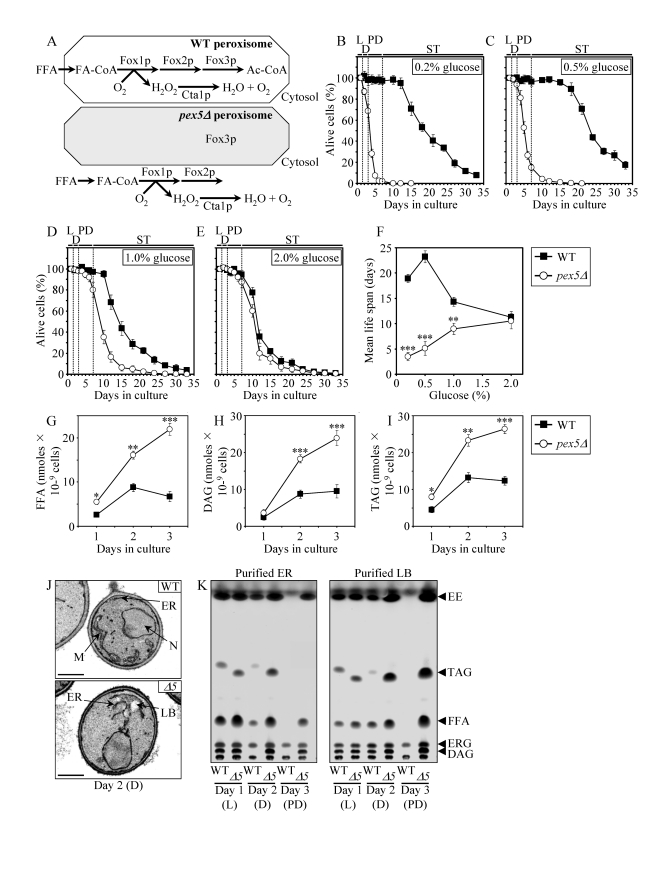
Figure 1. The
pex5Δ mutation shortens chronological life span (CLS), alters cell morphology and remodels lipid metabolism
in CR yeast. (A) Outline of subcellular
localization of the Fox1p, Fox2p and Fox3p enzymes of fatty acid
ß-oxidation in WT and pex5Δ cells. (B - F)
Survival and the mean life spans of chronologically aging WT and pex5Δ
yeast cultured in medium initially containing 0.2%, 0.5%, 1% or 2%
glucose. Data are presented as means ± SEM (n = 16-38; ***p < 0.001; **p
< 0.01). (G - I) Levels of free fatty acids
(FFA), diacylglycerols (DAG) and triacylglycerols (TAG) in WT and pex5Δ
cells grown on 0.2% glucose and taken for analyses at the indicated
time-points. FFA and TAG were measured by quantitative mass spectrometry.
The levels of DAG were quantitated by densitometric analysis of TLC plates.
Data are presented as means ± SEM (n = 3-8; ***p < 0.001; **p < 0.01;
*p < 0.05). (J and K)
Transmission electron micrographs (J) and spectra of lipids
extracted from purified endoplasmic reticulum (ER) and lipid bodies (LB)
and analyzed by TLC (K) for WT and pex5Δ (Δ5)
yeast grown on 0.2% glucose and taken for analyses at the
indicated time-points. Abbreviations: Cta1p, peroxisomal catalase; D,
diauxic growth phase; EE, ethyl esters; ERG, ergosterol; FA-CoA, CoA esters
of fatty acids; L, logarithmic growth phase; M, mitochondrion; N, nucleus;
PD, post-diauxic growth phase; ST, stationary growth phase.
In chronologically aging CR yeast, peroxisomal FFA
oxidation modulates,
perhaps via several negative feedback loops, the following three processes: 1)
the ER-confined biosynthesis of TAG from FFA and DAG; 2) the subsequent
deposition of TAG, the major neutral lipid reserves, in lipid bodies; and 3)
the consequent lipolysis of deposited TAG and the resulting formation of FFA
and DAG [39,40]. By impairing the
ability of peroxisomal FFA oxidation to act
as a rheostat that regulates cellular aging by modulating the
age-related dynamics of FFA, DAG and TAG in the ER and lipid bodies, the pex5Δ mutation caused the accumulation
of the closely apposed ER membranes and ER-originated lipid bodies in CR yeast
(Figure 1J). Of note, these morphological features of pex5Δ yeast under CR were similar to
those observed in a mouse model for the peroxisome biogenesis disorder
Zellweger syndrome with hepatocyte-specific elimination of the PEX5 gene
[51]. Furthermore, the pex5Δ
mutation increased the concentrations of FFA, DAG and TAG in CR yeast (Figures
1G to I), promoting their buildup in the ER and lipid bodies (Figure 1K). CR
yeast carrying the pex5Δ
mutation also accumulated the ER-derived and lipid bodies-deposited ergosteryl
esters (EE) neutral lipid species (Figure 1K).
Following
a short-term exposure to exogenous FFA (palmitoleic acid or oleic acid) or DAG,
wild-type (WT) cells grown under CR conditions died (Supplementary Figure 1A). The vast
majority of these WT cells displayed propidium iodide (PI) positive staining
characteristic of the loss of plasma membrane integrity, a hallmark event of
necrotic cell death (Supplementary Figure 1B and 1C). In contrast, only a minor portion of
these WT cells displayed Annexin V positive staining used to visualize the
externalization of phosphatidylserine, a hallmark event of apoptotic cell death
(Supplementary Figure 1B and 1C). Thus, a brief exposure of WT cells grown under CR
conditions to exogenous FFA or DAG caused their necrotic, not apoptotic, death.
Importantly, we found that the pex5Δ mutation enhances the susceptibility of
CR yeast to necrotic death caused by a short-term exposure to exogenous FFA or
DAG (Supplementary Figure 1A and 1C), perhaps due to the increased concentrations of
endogenous FFA and DAG seen in pex5Δ cells under CR (Figures 1G and H).
In
addition to its effect on lipid metabolism and lipid-induced necrotic cell
death, the pex5Δ mutation also
altered mitochondrial morphology and oxidation-reduction processes in
mitochondria of CR yeast. In fact, this mutation caused the fragmentation of a
tubular mitochondrial network into individual mitochondria under CR conditions
(Figures S2A and S2B). Furthermore, in CR
yeast the pex5Δ mutation 1)
greatly reduced the rate of oxygen consumption by mitochondria (Supplementary Figure 2C); 2)
substantially decreased the mito-chondrial membrane potential (Supplementary Figure 2D); and
3) diminished the level of intracellular ROS (Supplementary Figure 2E), known to be
generated mostly as by-products of mitochondrial respiration [10,52].
Interestingly, all these mitochondrial abnormalities in pex5Δ yeast under CR were reminiscent of changes in mitochondrial morpholo-gy
and functions seen in mice with hepatocyte-specific elimination of the PEX5
gene, a model for the peroxi-some biogenesis disorder Zellweger syndrome [51].
Besides
its profound effect on lipid metabolism, lipid-induced necrosis, mitochondrial
morphology and functions, the pex5Δ
mutation also 1) reduced the resistance of chronologically aging CR yeast to
chronic oxidative, thermal and osmotic stresses (Supplementary Figure 3A); 2) sensitized CR
yeast to death that was initiated in response to a short-term exposure to
exogenous hydrogen peroxide or acetic acid (Supplementary Figure 3B) and that is known to be
caused by mitochondria-controlled apoptosis [53,54]; and 3) elevated the
frequencies of deletion and point mutations in mitochondrial and nuclear DNA of
CR yeast (Figures S3C to S3E).
The
profound changes in cell morphology and physiology, stress resistance,
susceptibility to lipid-induced necrosis and mitochondria-controlled apoptosis,
and stability of nuclear and mitochondrial DNA seen in pex5Δ yeast under CR conditions
coincided with considerable changes in their proteome. Indeed, our mass spectrometry-based
quantitative proteomic analysis of proteins recovered in total cell lysates as
well as in purified ER and mitochondria revealed that the pex5Δ mutation altered the abundance
of many proteins (Figure 2A). Protein species that were depleted or enriched in
the total cell lysate, ER and mitochondria of pex5Δ yeast grown under CR conditions
included proteins involved in a number of cellular processes (Figure 2B).
Importantly, lack of 91 of these proteins increased the CLS of yeast under CR
(Figure 2B), suggesting their essential pro-aging role in longevity regulation
when calorie supply is limited. Noteworthy, 58 of the genes encoding these
proteins and termed gerontogenes (i.e., the genes whose mutant alleles
extend life span; [55]) have not been previously known as being critical for
defining the CLS of yeast. The identities of protein species that were depleted
or enriched in pex5Δ
yeast grown under CR conditions, the extent to which their levels were altered
and the names of gerontogenes identified in our functional analysis will be
reported elsewhere (Goldberg et al., manuscript in preparation). Importantly,
for most of these proteins (with
some exceptions, see Figures S4C and S4D) the fold increase or decrease in the
level of a protein enriched or depleted in pex5Δ was found to be in good correlation
with the fold increase or decrease (respectively) in the mean CLS of a mutant
strain lacking it (Figures S4A and S4B).
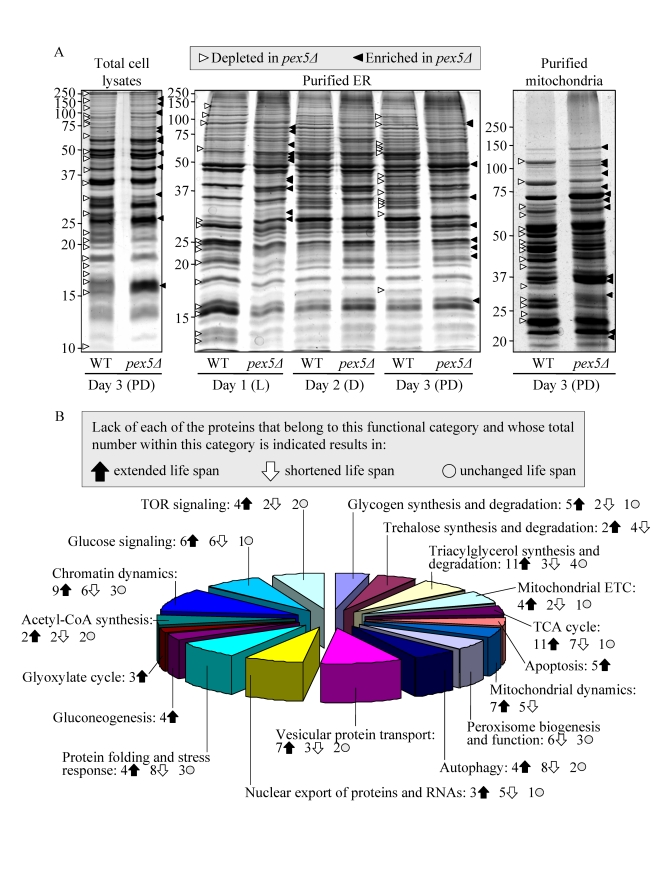
Figure 2. The pex5Δ mutation alters the abundance of many proteins recovered in total cell lysates, purified ER and mitochondria of CR yeast. (A) The spectra of
proteins recovered in total cell lysates, purified ER and mitochondria of
WT and pex5Δ cells that were grown under CR on 0.2% glucose and
taken for analyses at the indicated time-points. (B) Functional
categories of proteins that were enriched or depleted in the total cell
lysate, ER and mitochondria of pex5Δ cells (as compared to WT
cells) under CR conditions. Lack of 91 of these proteins increased the CLS
of yeast under CR, suggesting their essential pro-aging role in longevity
regulation when calorie supply is limited.
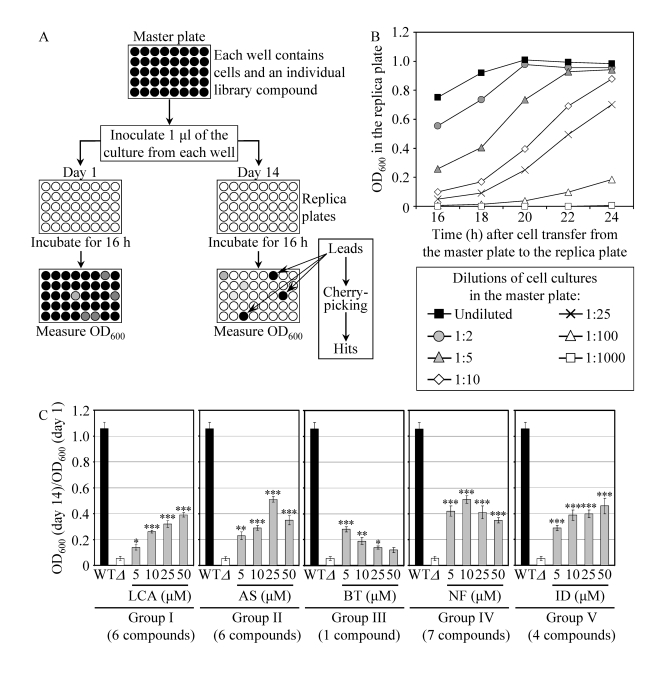
Figure 3. A high-throughput screen of compound libraries for small molecules that extend the CLS of yeast under CR conditions. (A) A microplate assay for
measuring yeast CLS by monitoring optical density at 600 nm (OD600)
was used for screening representative compounds from several commercial
libraries for small molecules that extend the CLS of pex5Δ cells grown
under CR on 0.5% glucose. (B) The OD600 of a cell culture
in the replica microplate following incubation for 16 to 24 hours
correlates with the number of viable cells present in this culture before
it was taken from the master microplate for replica plating. (C) The
effect of various concentrations of the identified anti-aging small
molecules on the CLS of the pex5Δ (Δ) strain under CR
conditions. The "OD600 at day 14/OD600 at day 1"
ratio was used as a measure of CLS. Data are
presented as means ± SEM (n = 3-5; ***p < 0.001; **p < 0.01; *p <
0.05). The anti-aging small molecules LCA, AS, BT, NF and ID belong
to five chemical groups.
Altogether,
these findings imply that, by impairing peroxisomal FFA oxidation and affecting
lipid metabolism in the ER and lipid bodies, the pex5Δ mutation alters the levels of
numerous pro- and anti-aging proteins and impacts many longevity-related processes,
thereby shortening the CLS of yeast when calorie supply is limited. We
therefore chose the short-lived pex5Δ strain to carry out a chemical genetic
screen for anti-aging compounds that target lipid metabolism to extend CLS in
yeast placed on a CR diet.
A
chemical genetic screen for small molecules that extend the CLS of yeast under
CR conditions
To
facilitate a high-throughput screen of
compound libraries for anti-aging small molecules, we adopted a previously
described microplate assay [56] for measuring CLS by monitoring optical density at 600 nm (OD600) (Figure 3A). In
our assay, a small aliquot of the pex5Δ culture grown in a nutrient-rich medium
containing 0.5% glucose and recovered from mid-logarithmic phase was
transferred into each well of a 96-well master microplate containing the same
growth medium and a compound from a commercially available library. At days 1,
7, 10 and 14 of the incubation of master microplates, a small aliquot of each
culture was transferred into individual wells of a new (replica) microplate
containing growth medium only. Following incubation of replica microplates for
16 hours, the OD600 of the culture in each well of the replica
microplate was measured. Importantly, we found that under such conditions the
OD600 of a cell culture in a well of the replica microplate
correlates with the number of viable cells in the corresponding well of the
master microplate (Figure 3B). To calculate survival at each time point, the OD600
at a particular time point was divided by the OD600 at day 1. By
translating our microplate assay into
high-throughput format and screening
representative compounds from the NIH Clinical Collection, Prestwick Chemical
Inc. and Sigma-LOPAC commercial libraries, we identified "lead" compounds. The
subsequent "cherry-picking" analysis of these small molecules revealed "hit" compounds
that in our microplate assay reproducibly extended the CLS of pex5Δ. Using the web-based eMolecules
searching engine, we identified commercially available structural analogs of
the hit compounds and then tested their life-extending efficacy in our microplate
assay for measuring the CLS of pex5Δ. By screening the total of
approximately 19,000 representative compounds from seven commercial libraries,
we identified 24 small molecules that greatly extend the CLS of pex5Δ under CR and belong to 5
chemical groups (Figure 3C). Group I consisted of 6 bile acids, including
lithocholic acid (LCA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA),
cholic acid (CA), dehydrocholic acid (DHCA) and hyodeoxycholic acid (HDCA)
(Figures 3C and S5). Noteworthy, the anti-aging efficacy of these bile acids
correlated with their hydrophobicity. In fact, LCA - the most hydrophobic bile
acid species [57] - displayed the highest ability to delay chronological aging
of pex5Δ under CR
conditions in the microplate assay (Supplementary Figure 5). The identities of small
molecules that belong to groups II to V (Figure 3C) of the anti-aging compounds
identified in our screen and the structure-activity analysis of their
life-extending potential will be reported elsewhere (Goldberg et al., manuscript
in preparation).
Noteworthy,
none of the small molecules that has been shown to extend CLS (i.e.,
caffeine, methionine sulfoximine, rapamycin and spermidine; Supplementary Table 1;
[56,58,59]) and/or RLS (i.e., rapamycin and resveratrol; Supplementary Table 1;
[27,31]) in yeast has been identified in our screen for compounds capable of
increasing the CLS of pex5Δ
under CR. Furthermore, none of these currently known life-extending molecules
is structurally related to the anti-aging compounds that we revealed. Thus, it
is likely that LCA and all other novel anti-aging compounds identified in our
screen target longevity-related cellular processes that are not modulated by
the presently known anti-aging small molecules. Because our screen was aimed at
identifying compounds that extend yeast longevity by targeting lipid
metabolism, it is conceivable that the age-related dynamics of TAG, FFA and DAG
is one of such cellular processes.
Pharmacophore
modeling of the anti-aging potential of bile acids
Similar
to their effect on pex5Δ,
some of the group I anti-aging compounds extended the CLS of WT strain under CR
conditions. Specifically, LCA and two other bile acids - DCA and CDCA -
increased both the mean and maximum CLS of WT yeast grown under CR on 0.2%
glucose (Figures 4A to 4D). Moreover, DHCA increased only the mean CLS of WT
yeast under CR at 0.2% glucose, whereas HDCA increased only their maximum CLS
(Figures 4A to 4D). Akin to its highest life-extending efficacy in pex5Δ under CR, the most hydrophobic
bile acid - LCA [57] - provided WT cells with the greatest longevity benefit
when calorie supply was limited. In fact, LCA increased the mean CLS of WT
strain under CR at 0.2% glucose by almost 250% and its maximum CLS by more than
200% (Figures 4A to D). Our comparative analysis of the structural differences
between various bile acids and their relative life-extending efficacies
revealed that the positions 6, 7 and 12 in the six-member rings B and C of the
steroid nucleus are important for the anti-aging potential of a bile acid.
Indeed, the ability of LCA to extend both the mean and maximum CLS of WT
yeast under CR can be: 1) eliminated (with respect to the mean CLS) or greatly
reduced (with respect to the maximum CLS) by attaching an α-oriented
hydroxyl group at the position 6 (as in HDCA); 2) greatly reduced (with respect
to both the mean and maximum CLS) by attaching an α-oriented hydroxyl
group at the position 7 (as in CDCA); and 3) greatly reduced (with respect to
both the mean and maximum CLS) by attaching an α-oriented hydroxyl group
at the position 12 (as in DCA) (Figures 4B to E). All these modifications to
the structure of LCA increase polarity of the hydrophilic (concave) side
[α-face] of the steroid nucleus by positioning a hydroxyl group below the
nucleus and axially to its plane (Figure 4E).
Furthermore, the anti-aging potential of LCA can be abolished by attaching a
β-oriented hydroxyl group at the position 7 (as in UDCA), thereby
conferring polarity to the hydrophobic (convex) side [β-face] of the
steroid nucleus by positioning a hydroxyl group above the nucleus and
equatorially to its plane (Figures 4B to E).
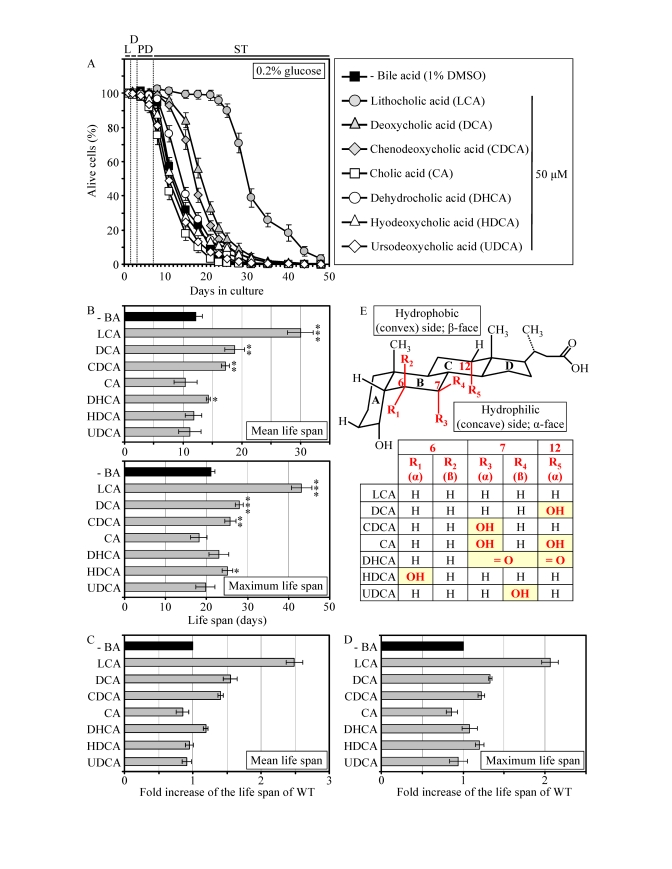
Figure 4. LCA and some other bile acids extend the CLS of WT strain under CR conditions. (A
- D) Effect of various bile acids on survival (A) and on the
mean and maximum life spans (B - D)
of chronologically aging WT strain grown under CR conditions on 0.2%
glucose. Data are presented as means ± SEM (n =
3-28; ***p < 0.001; **p < 0.01; *p < 0.05). (E)
Structure and hydrophilic/hydrophobic properties of bile acids. The R1
(α), R3 (α) and R5 (α) hydroxyl groups at the positions 6, 7
and 12 in the six-member rings B and C of the steroid nucleus
increase polarity of the hydrophilic (concave) side [α-face] of the
nucleus by being located below the nucleus and axially to its plane. The R4
(β) hydroxyl group at the position 7 in the six-member ring B of the
steroid nucleus confers polarity of the hydrophobic (convex) side
[β-face] of the nucleus by being located above the nucleus and
equatorially to its plane.
Moreover,
the simultaneous attachments of two α-oriented hydroxyl groups (as in CA)
or two keto groups (as in DHCA) at the positions 7 and 12 eliminated the
ability of LCA to extend both the mean and maximum CLS of WT yeast under
CR (Figures 4B to E). Altogether, the results of our pharmacophore modeling of
the anti-aging potential of bile acids imply that the maintenance of the
minimal polarity of both the hydrophilic (concave) and hydrophobic (convex)
sides of the steroid nucleus - by avoiding the presence of polar substituents
at the positions 6, 7 and 12 - is mandatory for the extreme life-extending
efficacy of LCA under CR conditions. Such stringent structural requirements are
consistent with a target specificity of LCA action as an anti-aging small
molecule.
LCA extends the CLS of WT yeast under both CR and non-CR
conditions, although to a different extent
If
added to growth medium at the time of cell inoculation, LCA increased both the
mean and maximum CLS of WT strain not only under CR at 0.2% or 0.5% glucose
(Figures 5A, 5B and 5G - 5I) but also under non-CR conditions administered by
culturing yeast in medium initially containing 1% or 2% glucose (Figures 5C, 5D
and 5G - 5I). At any tested concentration of glucose in growth medium, LCA
displayed the greatest beneficial effect on both the mean and maximum CLS of WT
strain if used at a final concentration of 50 μM (Figures 5E and 5F). It
should be stressed that the life-extending efficacy of 50 μM LCA under CR
exceeded that under non-CR conditions, being inversely proportional to the
concentration of glucose in growth medium and thus in correlation with the
extent of calorie supply limitation (Figures 5G to 5I). Importantly, although
50 μM LCA displayed a profound effect on CLS, it did not cause significant
changes in growth of WT strain at any tested concentration of glucose in
medium. In fact, both growth rate in logarithmic phase and time prior to entry
into stationary (ST) phase were similar for WT cells cultured in medium with or
without LCA (Supplementary Figure 6).
LCA
extends the CLS of WT yeast under CR by modulating a compendium of
longevity-related processes
Our chemical genetic screen identified
LCA as a compound that under CR conditions extends the CLS of pex5Δ, a prematurely aging mutant strain displaying profound changes in
lipid metabolism, lipid-induced necrotic cell death, mitochondrial morphology
and functions, stress resistance, mitochondria-controlled apoptosis, and
stability of nuclear and mitochondrial DNA. We found that LCA also greatly
increases the mean and maximum CLS of WT yeast limited in calorie supply. This
finding prompted us to investigate how the exposure of WT cells to LCA under CR
conditions influences a compendium of longevity-related processes impaired in pex5Δ.
Consistent
with its sought-after effect on lipid metabolism in the ER, lipid bodies and
peroxisomes, LCA elevated the concentration of TAG in WT cells that entered the
non-proliferative ST phase under CR at 0.2% glucose (Figure 6A). Furthermore,
under these conditions LCA also substantially reduced the intracellular levels
of FFA and DAG in WT yeast that reached reproductive maturation by entering
into ST phase (Figures 6B and 6C). Moreover, LCA greatly reduced the susceptibility
of reproductively mature WT cells under CR to necrotic cell death that was
caused by a short-term exposure to exogenous FFA or DAG and defined by Annexin
V-/PI+ staining (Figures 6D to 6I).
The exposure of reproductively mature WT cells to LCA under CR conditions also
influenced other longevity-related processes impaired in pex5Δ, including those confined to mitochondria. Indeed, in WT cells that
entered the non-proliferative ST phase under CR at 0.2% glucose, LCA 1)
attenuated the fragmentation of a tubular mitochondrial network into individual
mitochondria (Figure 7A); 2) elevated the rate of oxygen consumption by
mitochondria (Figure 7B); 3) reduced the mitochondrial membrane potential
(Figure 7C); and 4) decreased the level of intracellular ROS (Figure 7D) known
to be generated mainly in mitochondria [10,52].
Moreover,
in WT yeast that under CR conditions reached reproductive maturation by
entering into ST phase, LCA 1) enhanced cell resistance to oxidative and
thermal (but not to osmotic) stresses (Figure 7E); 2) reduced cell
susceptibility to death triggered by a short-term exposure to exogenous
hydrogen peroxide or acetic acid (Figure 7F) known to be caused by
mitochondria-controlled apoptosis [53,54]; and 3) decreased the frequencies of deletions
and point mutations in mitochondrial and nuclear DNA (Figures 7G to I).
LCA
extends yeast CLS independent of TOR, by modulating housekeeping longevity
assurance pathways
Our
chemical genetic screen was aimed at identifying small molecules that can
increase the CLS of yeast under CR by modulating housekeeping longevity
pathways. Such pathways may regulate yeast longevity irrespective of the number
of available calories and may not necessarily overlap (or may only partially
overlap) with the adaptable longevity pathways that are under the stringent
control of calorie availability. In chronologically aging yeast, the TOR and
cAMP/PKA signaling
pathways are the two adaptable longevity pathways that govern the life-extending
effect of CR (Figure 10A) [5,6,60-62].
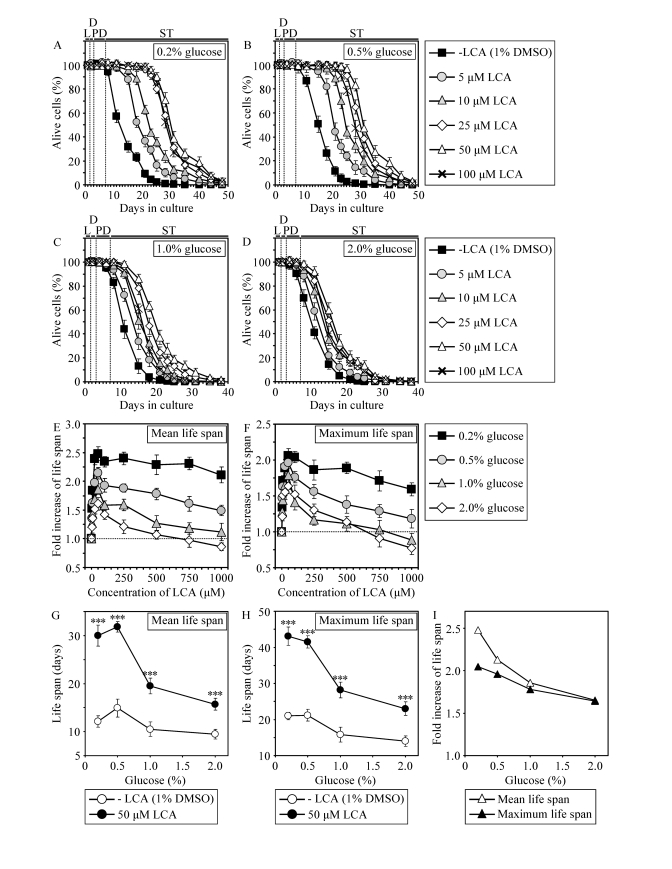
Figure 5. In chronologically aging WT yeast, the life-extending efficacy of LCA under CR exceeds that under non-CR conditions. (A - F)
Effect of various concentrations of LCA on survival (A - D)
and on the fold increase in the mean (E) or
maximum (F) life span of chronologically aging WT strain
cultured in medium initially containing 0.2%, 0.5%, 1% or 2% glucose. Data are presented as means ± SEM (n = 3-28). (G
- I) Effect of 50 μM LCA on the mean or
maximum CLS of WT yeast cultured in
medium initially containing 0.2%, 0.5%, 1% or 2% glucose. Data are presented as means ± SEM (n = 12-28; ***p
< 0.001).
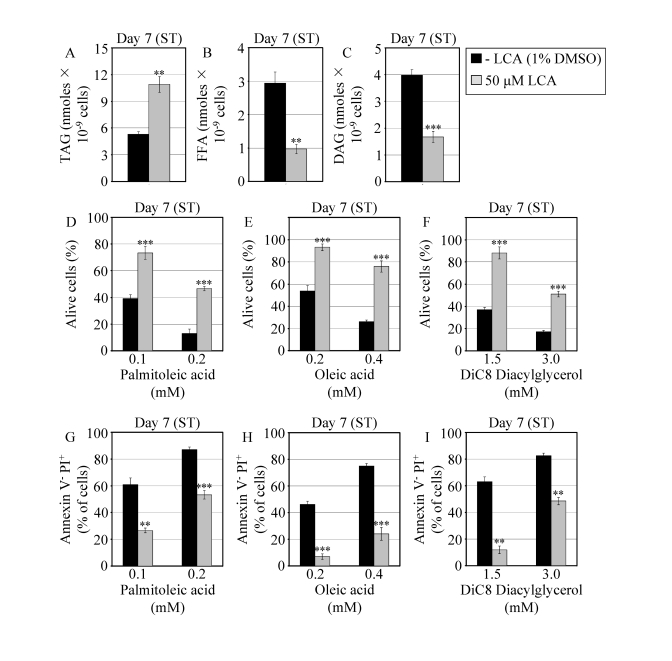
Figure 6. In chronologically aging WT yeast that entered the non-proliferative stationary (ST) phase under CR, LCA alters the levels of lipids and protects cells from lipid-induced necrotic death. (A - C)
Levels of triacylglycerols (TAG) and free fatty acids (FFA) measured by
quantitative mass spectrometry (A and B, respectively) and of
diacylglycerols (DAG) monitored by TLC (C) in WT cells grown in
medium with or without LCA. (D - F) Viability of WT cells
pre-grown in medium with or without LCA and then treated for 2 h with
palmitoleic acid (D), oleic acid (E) or DiC8 diacylglycerol (F).
(G - I) Percent of WT cells (pre-grown in medium with or
without LCA) that following their treatment with palmitoleic acid (G),
oleic acid (H) or DiC8 diacylglycerol (I) displayed Annexin V
negative and PI positive (Annexin V- and PI+)
staining characteristic of necrotic cell death. Data are presented as means
± SEM (n = 3-9; ***p < 0.001; **p < 0.01).
WT cells grown on 0.2% glucose in the presence or absence of LCA were taken
for analyses at day 7, when they reached reproductive maturation by
entering into ST phase.
Reduction of the Tor1p protein kinase
activity in yeast placed on a CR diet or exposed to rapamycin prevents
inhibitory phosphorylation of Atg13p, a key positive regulator of autophagy,
thereby activating this essential anti-aging process (Figure 10A) [63,64].
Under CR conditions or in response to rapamycin, Tor1p is also unable to
phosphorylate and activate the nutrient-sensory protein kinase Sch9p [60,65].
The resulting inhibition of the Sch9p kinase activity suppresses its ability to
attenuate protein synthesis in mitochondria, thus turning on this essential
anti-aging process [61].
Furthermore,
by inhibiting the Sch9p kinase activity, CR restrains Sch9p from activating
protein synthesis in the cytosol, thereby slowing down this essential pro-aging
process [60,62,65]. Moreover, the attenuation of the Sch9p kinase activity in
CR yeast prevents the retention of Rim15p in the cytosol, hence allowing this
nutrient-sensory protein kinase to enter the nucleus where it orchestrates an
anti-aging transcriptional program by activating the stress response
transcriptional activators Msn2p, Msn4p and Gis1p [58,62]. The longevity
benefit associated with CR in chronologically aging yeast is also due to the
attenuation of signaling through the cAMP/PKA pathway, which is driven by
glucose deprivation [5,6,62]. By preventing inhibitory phosphorylation of
Atg13p, the reduction of the PKA kinase activity in CR yeast results in
activation of autophagy (Figure 10A) [63,66]. In addition, by inhibiting the
PKA kinase activity, CR suppresses the ability of PKA to activate protein
synthesis in the cytosol [62]. Moreover, reduced PKA kinase activity in CR
yeast enables nuclear import of Msn2p and Msn4p, thus
turning on an anti-aging transcriptional program driven - in a Rim15p-dependent
fashion - by these two transcriptional activators [27,62,67]. Noteworthy, the
kinase activity of the cytosolic pool of Rim15p is inactivated through
PKA-dependent phosphorylation (Figure 10A) [62]. Although some of the Rim15p
phosphorylation targets are involved in longevity regulation and reside outside
the nucleus [68], a role of such phosphorylation in the life-extending effect
of CR in yeast remains to be established.
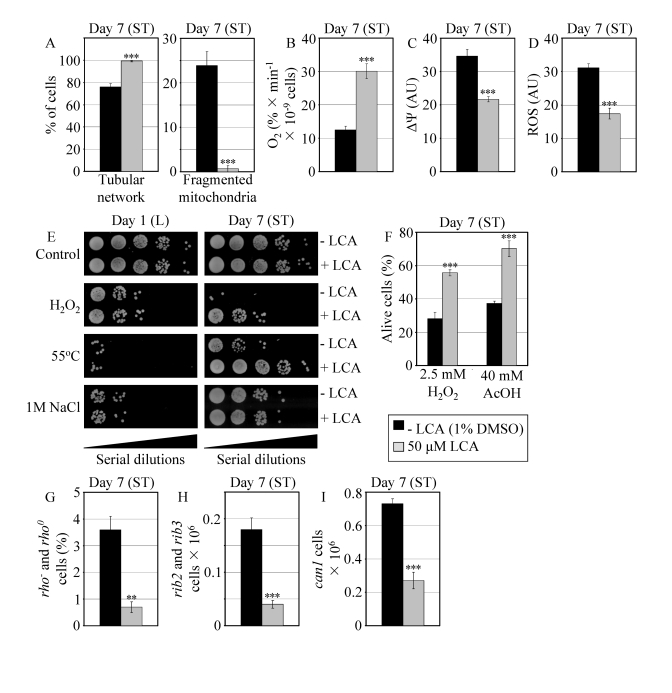
Figure 7. In reproductively mature WT yeast that entered the non-proliferative stationary (ST) phase under CR, LCA modulates mitochondrial morphology and functions, enhances stress resistance, attenuates mitochondria-controlled apoptosis, and increases stability of nuclear and mitochondrial DNA. (A)
Percent of WT cells grown in medium with or without LCA and exhibiting a
tubular mitochondrial network or fragmented mitochondria. Mitochondria were
visualized by indirect immunofluorescence microscopy using monoclonal
anti-porin primary antibodies and Alexa Fluor 568-conjugated goat
anti-mouse IgG secondary antibodies. (B - D) Oxygen
consumption by WT cells grown in medium with or without LCA (B),
their mitochondrial membrane potential ΔΨ (C) and their
ROS levels (D). ΔΨ and ROS were visualized in living cells by
fluorescence microscopy using fluorescent dyes Rhodamine 123 or
Dihydrorhodamine 123, respectively. (E) The resistance of WT cells
pre-grown in medium with or without LCA to chronic oxidative, thermal and
osmotic stresses. (F) Viability of WT cells pre-grown in medium with
or without LCA and then treated for 1 h with hydrogen peroxide or acetic
acid (AcOH) to induce mitochondria-controlled apoptosis. (G- I)
The frequencies of rho- and rho0mutations
in mitochondrial DNA (G), rib2 and rib3 mutations in
mitochondrial DNA (H), and of can1 (Canr)
mutations in nuclear DNA (I) of WT cells grown in medium with or without
LCA. Data in A - D and F - I are presented as
means ± SEM (n = 4-17; ***p < 0.001; **p <
0.01). WT cells grown on 0.2% glucose in the presence or absence of
LCA were taken for analyses at day 7, when they reached reproductive
maturation by entering into ST phase.
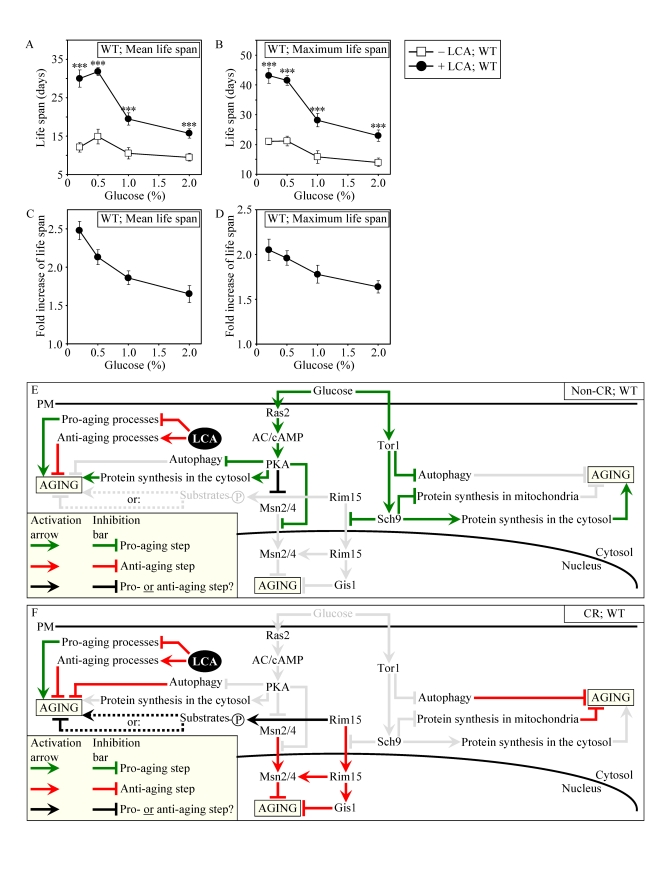
Figure 8. LCA increases the CLS of WT strain to the highest extent under CR conditions. (A and B)
Effect of LCA on the mean (A) and maximum (B) life spans of
chronologically aging WT strain. Data are
presented as means ± SEM (n = 12-28; ***p < 0.001). (C - E) Effect of LCA on the
fold increase in the mean (C) or maximum (D)
life span of chronologically aging WT strain. Data are presented as means ± SEM (n = 12-28). Cells
in A to D were cultured in medium initially containing 0.2%,
0.5%, 1% or 2% glucose in the presence of LCA (50 μM) or in its
absence. Survival data are provided in Supplementary Figure 9. (E
and F) Outline of pro- and anti-aging processes that are controlled
by the TOR and/or cAMP/PKA signaling pathways and are modulated by LCA in
WT cells grown under non-CR (E) or CR (F) conditions.
Activation arrows and inhibition bars denote pro-aging processes (displayed
in green color), anti-aging processes (displayed in red color) or processes
whose role in longevity regulation is presently unknown (displayed in black
color). Doted lines denote hypothetical processes. Abbreviations:
PM, plasma membrane.
Our
evaluation of the life-extending efficacy of LCA in WT strain on a high- or
low-calorie diet revealed that this compound increased CLS irrespective of the
number of available calories (Figures 8A and 8B). Intriguingly, the extent to
which LCA extended longevity was highest under CR conditions (Figures 8C and
8D), when the pro-aging processes modulated by the adaptable TOR and cAMP/PKA
pathways are suppressed and the anti-aging processes are activated (Figure 8F).
The life-extending efficacy of LCA in CR yeast significantly exceeded that in
yeast on a high-calorie diet (Figures 8C and 8D), in which the adaptable TOR
and cAMP/PKA pathways greatly activate the pro-aging processes and suppress the
anti-aging processes (Figure 8E). Altogether, these findings suggest that,
consistent with its sought-after effect on a longevity signaling network, LCA
mostly targets certain housekeeping longevity assurance pathways that do not
overlap (or only partially overlap) with the adaptable TOR and cAMP/PKA
pathways modulated by calorie availability (Figures 8E and 8F).
Consistent
with our assumption that LCA extends longevity not by modulating the adaptable
TOR pathway (Figures 9E and 9F), lack of Tor1p did not impair the
life-extending efficacy of LCA under CR (Figures 9A to 9D). Importantly, by
eliminating a master regulator of this key adaptable pathway that shortens the
CLS of yeast on a high-calorie diet, the tor1Δ mutation abolished the
dependence of the anti-aging efficacy of LCA on the number of available
calories. In fact, LCA extended longevity of the tor1Δ mutant strain to a very similar
degree under CR and non-CR conditions (Figures 9C and 9D).
We
next assessed how the adaptable cAMP/PKA pathway influences the life-extending
efficacy of LCA in yeast on a high- or low-calorie diet. Although the ras2Δ mutation greatly decreases the
PKA protein kinase activity by eliminating a GTP-binding protein that activates
adenylate cyclase responsible for the synthesis of the PKA activator cAMP
(Figures S7E and S7F) [62], it did not abolish the ability of LCA to extend CLS
under CR and non-CR conditions (Figures S7A and S7B). However, the
life-extending efficacy of LCA was decreased by the ras2Δ mutation, as compared to that
seen in WT cells exposed to this compound (Figures S7C and S7D). In spite of
such partial reduction of the anti-aging potential of LCA in ras2Δ, LCA still significantly
increased its CLS under CR and non-CR conditions (Figures S7C and S7D).
Thus,
it seems that LCA extends longevity of chronologically aging yeast through two
different mechanisms. Firstly, irrespective of the number of available
calories, this bile acid targets certain house-keeping longevity assurance
pathways that 1) inhibit some pro-aging processes and/or activate some
anti-aging processes; and 2) do not overlap with the adaptable cAMP/PKA pathway
modulated by calorie availability (Figure 10B). Secondly, we propose that LCA
unmasks the anti-aging potential of PKA by activating PKA-dependent
phosphorylation of the cytosolic pool of Rim15p (Figure 10B). Because such
phosphorylation of Rim15p is known to inactivate its protein kinase activity
(Figure 10A) [62], we hypothesize that, while the nuclear pool of Rim15p has a
well established anti-aging function [5,6,27,62], the cytosolic pool of this
nutrient-sensory protein kinase plays an essential pro-aging role by
phosphorylating a compendium of proteins that promote aging only if
phosphorylated (Figure 10B). Noteworthy, some of the Rim15p phosphorylation
targets are involved in longevity regulation
and reside outside the nucleus [68]. In our hypothesis, LCA can unmask the
anti-aging potential of PKA only when PKA is activated by cAMP, i.e.,
under non-CR conditions (Figures 8E and F). Consistent with our hypothesis on
the two mechanisms underlying the anti-aging effect of LCA, lack of Ras2p only
partially and to the same extent reduced the life-extending potential of LCA
under both CR and non-CR conditions (Figures S7C and S7D), likely by impairing
the mechanism in which LCA unmasks the anti-aging potential of PKA. The
resulting inability of PKA to inhibit the proposed pro-aging role of the
cytosolic pool of Rim15p in ras2Δ cells would make the Rim15p-dependent pro-aging mechanism
constitutively active in these cells, regardless of the number of available
calories or presence of LCA (Figures S7E and S7F).
The TOR and cAMP/PKA
pathways converge on Rim15p whose nuclear pool plays a pivotal role in
governing the life-extending effect of CR by enabling the establishment of an
anti-aging transcriptional program driven by Msn2p, Msn4p and Gis1p (Figure 10A) [5,6,27,62]. Our evaluation of the life-extending efficacy of LCA in yeast
lacking Rim15p further supported the notion that one of the two mechanisms
underlying the anti-aging effect of this bile acid involves its ability to
modulate certain housekeeping longevity assurance pathways that are not
centered on Rim15p and do not overlap with the adaptable TOR and cAMP/PKA
pathways. In fact, although the life-extending potential of LCA in rim15Δ was partially reduced (Figures S8C and S8D) due to the impairment of
the Rim15p-centered mechanism of its anti-aging action (Figures S8E and S8F),
LCA still significantly increased the CLS of rim15Δ under CR and non-CR conditions (Figures S8A to S8D). Importantly, by
eliminating a key nutrient-sensory protein kinase on which the adaptable TOR
and cAMP/PKA pathways converge to regulate longevity in a calorie
availability-dependent fashion, the rim15Δ mutation abolishedthe dependence of the anti-aging efficacy of LCA on the number of available calories
(Figures S8C and S8D).
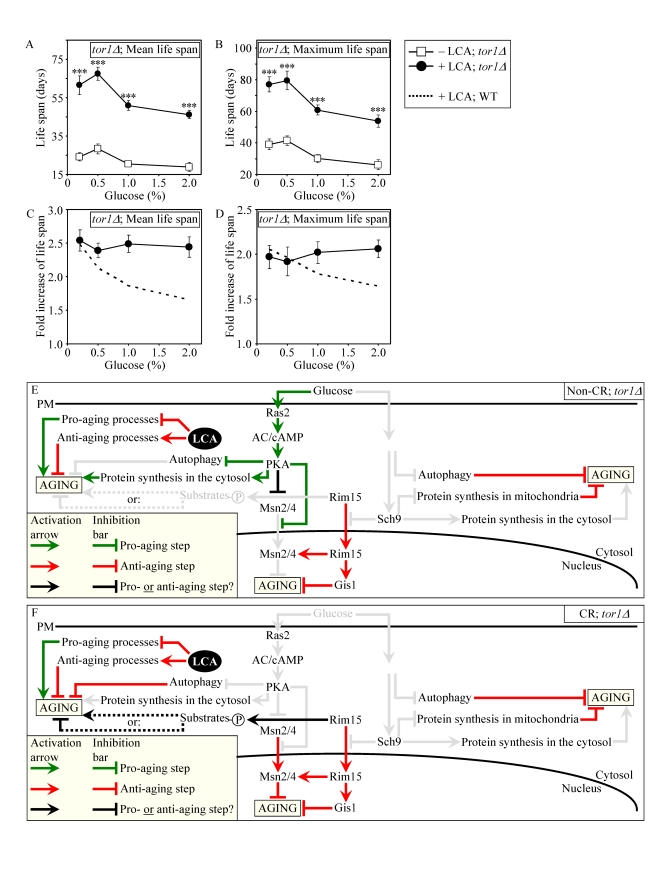
Figure 9. Lack of Tor1p does not impair the life-extending effect of LCA and abolishes the dependence of the anti-aging efficacy of LCA on the number of available calories. (A
and B) Effect of LCA on the mean (A) and maximum (B)
life spans of chronologically aging tor1Δ strain. Data are presented as means ± SEM (n = 4-7; ***p
< 0.001). (C and D) Effect
of LCA on the fold increase in the mean (C) or
maximum (D) life spans of chronologically aging tor1Δ and WT strains.
Data are presented as means ± SEM (n = 4-7). Cells
in A to D were cultured in medium initially containing 0.2%,
0.5%, 1% or 2% glucose in the presence of LCA (50 μM) or in its
absence. Survival data are provided in Supplementary Figure 10. (E
and F) Outline of pro- and anti-aging processes that are controlled
by the TOR and/or cAMP/PKA signaling pathways and are modulated by LCA in tor1Δcells grown under non-CR (E) or CR (F)
conditions.
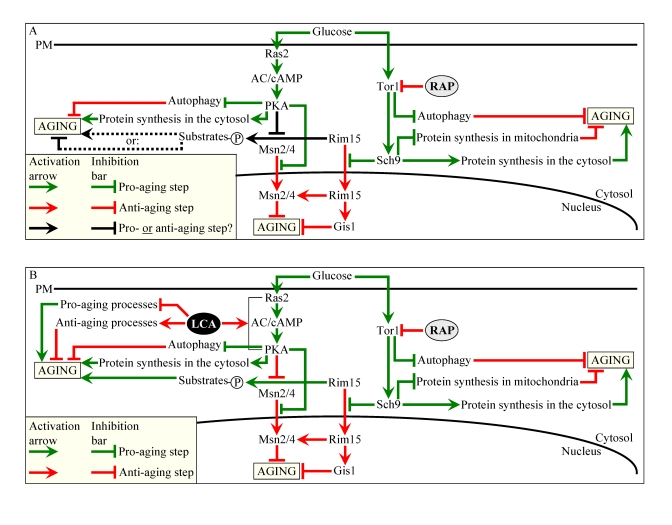
Figure 10. Outline of pro- and anti-aging processes that are controlled by the TOR and/or cAMP/PKA signaling pathways and are modulated by LCA or rapamycin (RAP) in chronologically aging yeast. The
currently accepted (A) and updated, based on this study (B),
outlines of pro- and anti-aging processes are shown. Activation
arrows and inhibition bars denote pro-aging processes (displayed in green
color), anti-aging processes (displayed in red color) or processes whose
role in longevity regulation was unknown (displayed in black color). Doted
lines denote hypothetical, until this study, processes. See text for
details.
Discussion
In this study, we designed a chemical
genetic screen for small molecules that increase the CLS of yeast under CR
conditions by targeting lipid metabolism and modulating housekeeping longevity
pathways that regulate longevity irrespective of the number of available
calories. Our screen identifies LCA as one of such molecules. Our analysis of
how LCA influences various longevity-related processes and how it affects the CLS
of yeast mutants impaired in the adaptable TOR and cAMP/PKA longevity pathways
provided important new insights into mechanisms of longevity regulation, as
outlined below.
LCA
extends yeast CLS by modulating housekeeping longevity assurance processes that
are not regulated by the adaptable TOR and cAMP/PKA signaling pathways
Our
findings imply that LCA extends longevity of chronologically aging yeast by
targeting two different mechanisms. One mechanism extends longevity regardless
of the number of available calories. This mechanism involves the LCA-governed
modulation of certain housekeeping longevity assurance pathways that do not
overlap with the adaptable TOR and cAMP/PKA pathways (Figure 10B). We identify
a compendium of processes that compose LCA-targeted housekeeping longevity
assurance pathways. Our data provide evidence that LCA modulates these pathways
by 1) suppressing the pro-aging process [39,40,50] of lipid-induced necrotic
cell death, perhaps due to its observed ability to reduce the intracellular
levels of FFA and DAG that trigger such death; 2) attenuating the pro-aging
process [69,70] of mitochondrial fragmentation, a hallmark event of age-related
cell death; 3) altering oxidation-reduction processes in mitochondria - such as
oxygen consumption, the maintenance of membrane potential and ROS production -
known to be essential for longevity regulation [8,10,11,71]; 4) enhancing cell
resistance to oxidative and thermal stresses, thereby activating the anti-aging
process [11,39,40,72,73] of stress response; 5) suppressing the pro-aging
process [69,70] of mitochondria-controlled apoptosis; and 6) enhancing
stability of nuclear and mitochondrial DNA, thus activating the anti-aging
process [74,75] of genome maintenance. The observed pleiotropic effect of LCA
on a compendium of housekeeping longevity assurance processes implies that this
bile acid is a multi-target life-extending compound that increases CLS in yeast
by modulating a network of the highly integrated processes that are not
controlled by the adaptable TOR and cAMP/PKA pathways. The major challenge now
is to define the molecular mechanisms by which LCA modulates each of these pro-
and anti-aging housekeeping processes and integrates them in chronologically
aging yeast.
The other mechanism underlying the
life-extending effect of LCA in chronologically aging yeast increases life span
only under non-CR conditions. This mechanism consists in LCA-driven unmasking
of the previously unknown anti-aging potential of PKA, a key player in the
adaptable cAMP/PKA pathway. We propose that LCA unveils the anti-aging
potential of PKA by activating PKA-dependent phosphorylation of the cytosolic
pool of Rim15p, a key nutrient-sensory protein kinase on which the adaptable
TOR and cAMP/PKA pathways converge to regulate longevity in a calorie
availability-dependent fashion (Figure 10B). Of note, the nuclear pool of
Rim15p is well known for its anti-aging role in governing the life-extending
effect of CR by enabling a pro-longevity transcriptional program driven by
Msn2p, Msn4p and Gis1p (Figure 10B) [6,62]. In our hypothesis 1) unlike its
nuclear pool, the cytosolic pool of Rim15p has an essential pro-aging function
in phosphorylating a compendium of its cytosolic target proteins [68] some of
which promote aging only if phosphorylated (Figure 10B); 2) under non-CR
conditions LCA activates the PKA-dependent phosphorylation of Rim15p (Figure 10B); and 3) because the phosphorylation of Rim15p inactivates its protein
kinase activity [62], the dephosphorylation of pro-aging target proteins of
Rim15p in the cytosol by phosphatases inhibits the ability of these target
proteins to promote aging (Figure 10B). To test the validity of our hypothesis,
we are currently evaluating how genetic manipulations that alter the abundance
of various extra-nuclear target proteins of Rim15p or affect their
phosphorylation status influence the life-extending efficacy of LCA.
Bile acids are beneficial to health and longevity across phyla
It
should be stressed that, although we found that LCA greatly extends yeast
longevity, yeast do not synthesize this or any other bile acid found in mammals
[57,76]; our mass spectrometry-based analysis of the total yeast lipidome has
confirmed lack of endogenous bile acids. One could envision that during
evolution yeast have lost the ability to synthesize bile acids but have
maintained the life-extending response to these biologically active molecules
by retaining certain longevity-related processes that are sensitive to
regulation by bile acids. Alternatively, one could think that during evolution
yeast have developed the ability to sense bile acids produced by mammals
(and/or bile acid-like lipids synthesized by worms), recognize these mildly
toxic molecules as environmental stressors providing hormetic benefits and/or
as indicators of the state of the environment or food supply, and then to
respond by undergoing certain life-extending changes to their physiology that
ultimately increase their chances of survival. It is conceivable therefore that
the life-extending potential of LCA and other bile acids as well as, probably,
the mechanisms underlying their anti-aging action are evolutionarily conserved.
In
fact, following their synthesis from cholesterol in the intestine, hypodermis,
spermatheca and sensory neurons of worms, bile acid-like dafachronic acids
(including 3-keto-LCA) are delivered to other tissues where they activate the
DAF-12/DAF-16 signaling cascade that in turn orchestrates an anti-aging
transcriptional program, thereby increasing the life span of the entire
organism [41]. Bile acids also provide health
benefits to mammals. Synthesized from cholesterol in hepatocytes of the liver,
these amphipathic molecules have been for a long time considered to function
only as trophic factors for the enteric
epithelium and as detergents for the emulsification and absorption of dietary
lipids and fat-soluble vitamins [57,76,77]. Recent years have been marked by a
significant progress in our understanding of the essential role that bile acids
play as signaling molecules regulating lipid, glucose and energy homeostasis
and activating detoxification of xenobiotics [57,77,78]. By stimulating the
G-protein-coupled receptor TGR5, bile acids activate the cAMP/PKA signaling
pathway that 1) enhances energy expenditure in brown adipose tissue and muscle
by stimulating mitochondrial oxidative phosphorylation and un-coupling; 2)
improves liver and pancreatic function by activating the endothelial nitric
oxide synthase; and 3) enhances glucose tolerance in obese mice by inducing
intestinal glucagon-like peptide-1 release [57,76,78]. Furthermore, by
activating the farnesoid X receptor (FXR) and several other nuclear hormone
receptors inside mammalian cells, bile acids 1) modulate the intracellular
homeostasis of cholesterol, neutral lipids and fatty acids; 2) regulate glucose
metabolism by enhancing glycogenesis and attenuating gluconeo-genesis; and 3)
stimulate clearance of xenobiotic and endobiotic toxins by activating
transcription of numerous xenobiotic detoxification genes [57,76-78]. All these
health-improving, beneficial metabolic effects of bile acids prevent the
development of obesity following administration of high-fat diet [57,76,77].
Thus, bile acids have a great potential as pharmaceutical agents for the
treatment of diabetes, obesity and various associated metabolic disorders, all
of which are age-related [57,76]. Moreover, bile acids have been shown to inhibit
neuronal apoptosis in experimental rodent models of neurodegenerative disorders
by promoting mitochondrial membrane stability, preventing the release of
cytochrome c from mitochondria, reducing activities of various caspases, and
activating the NF-κB, PI3K and MAPK survival pathways [79,80].
It should be stressed that many of the metabolic,
stress response and apoptotic processes modulated by bile acids in mammals are
essential for healthy aging and longevity regulation. Importantly, we found that, by modulating several of these
health- and longevity-related processes in chronologically aging yeast,
LCA increases their life span. Moreover, the long-lived Ghrhrlit/lit
mice displayed elevated levels of several bile acids and exhibited increased FXR-dependent transcription of numerous xenobiotic detoxification genes; if
administered to food consumed by wild-type mice, cholic acid - one of these
bile acids - mimicked the FXR-governed gene expression pattern observed in
Ghrhrlit/lit mice [81,82]. It
has been therefore proposed that, by promoting chemical hormesis in
mammals, these mildly toxic molecules with detergent-like properties may extend
their longevity by acting as endobiotic regulators
of aging [73,82,83].
Altogether, these
findings support the notion that bile acids act as endobiotic and xenobiotic regulators of aging that are
beneficial to health and longevity across phyla. A comparative analysis of the
mechanisms underlying such health-improving
and life-extending action of bile acids
implies that these mechanisms are likely to be evolutionarily conserved.
Methods
Yeast
strains and growth conditions.
The WT strain BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and single-gene-deletion mutant strains in the
BY4742 genetic background (all from Open Biosystems) were grown in YP medium
(1% yeast extract, 2% peptone) containing 0.2% 0.5%, 1% or 2% glucose as carbon
source. Cells were cultured at 30oC with rotational shaking at 200
rpm in Erlenmeyer flasks at a "flask volume/medium volume" ratio of 5:1.
Chemical
genetic screen for compounds that increase chronological life span (CLS).
The screen was
conducted at the High Throughput/Content Screening Facility at McGill
University. The single-gene-deletion mutant strain pex5Δ (MATα his3Δ1
leu2Δ0 lys2Δ0 ura3Δ0 pex5Δ::kanMX4) from Open Biosystems was grown in YPA0.5D medium (1%
yeast extract, 2% peptone, 50 μg/ml ampicillin, 0.5% glucose). 3-μl aliquots of
the pex5Δculture
recovered from mid-logarithmic phase at a cell titre of 2 Í 107 cells/ml were aliquoted into 96-well
master microplates using a Beckman Coulter high density Biomek FXII replica
pinning robot. Each well of a master microplate contained 96 μl of YPA0.5D
medium. 1 μl of a compound stock solution from a commercially available library
(each compound at 5 mM in dimethylsulfoxide (DMSO)) was added to each well
using a Beckman Coulter high density Biomek FXII replica pinning robot. Wells
of a master microplate supplemented with 1% DMSO (1 μl of DMSO per a well
containing 3 μl of the pex5Δculture and 96 μl of YPA0.5D medium) were used as
negative controls. Each master plate was created in duplicate. The master
microplates were sealed and incubated without shaking at 30oC in a
moist chamber. At days 1, 7, 10 and 14 of the
incubation of master microplates, a 3-μl aliquot of each culture was
transferred into individual wells of a new (replica) microplate containing 97
μl of YPA0.5D medium. Following incubation of sealed replica microplates in a
moist chamber for 16 hours at 30oC (to allow for growth of cells that were still
viable), the optical density at 600 nm (OD600) of the culture in each well of
the replica microplate was measured using a Molecular Devices Analyst HT
plate reader. To calculate survival at each time point, the OD600 at a
particular time point was divided by the OD600 at day 1. "Cherry-picking" of
the identified "lead" com-pounds for possible "hits" was carried out as
described above, with each lead compound being used at a final concentration of 5, 10, 25 or 50
μM and assessed in triplicate for validation. Commercially available structural
analogs of hit compounds were identified using the web-based eMolecules
searching engine. In total, approximately 19,000 representative compounds from
the BIOMOL, Chembridge, Maybridge, MicroSource Discovery, NIH Clinical
Collection, Prestwick Chemical Inc. and Sigma-LOPAC commercial libraries were
tested using the screen for chemical modulators of longevity.
Pharmacological manipulation of
CLS.
CLS analysis was performed as
previously described [39]. The chenodeoxycholic (C9377), cholic (C1129),
dehydrocholic (D3750), deoxycholic (D2510), hyodeoxycholic (H3878), lithocholic
(L6250) and ursodeoxycholic (U5127) bile acids were from Sigma. Their stock
solutions in DMSO were made on the day of adding each of these compounds to
cell cultures. Compounds were added to growth medium at the indicated
concentration immediately following cell inoculation. The final concentration
of DMSO in yeast cultures supplemented with a bile acid (and in the
corresponding control cultures supplemented with drug vehicle) was 1% (v/v).
Miscellaneous procedures.
Fluorescence
[39], immuno-fluorescence [39] and electron [84] microscopies followed by
morphometric analyses of the resulting images have been described elsewhere.
Extraction of lipids and their separation, identification and quantitation with
the help of TLC were performed according to established procedures [84]. Mass
spectrometric identification and quantitation of various lipid species were
carried as previously described [85]. Subcellular fractionation and organelle
purification,cell viability and stress resistance assays, oxygen consumption assay,
the measurement of the frequencies of spontaneous point and deletion mutations
in mitochondrial and nuclear DNA, total cell lysates preparation, and mass
spectrometric identification and quantitation of proteins were performed
according to established procedures [39].
This work was
supported by grants from the CIHR, NSERC of Canada, Canada
Foundation for Innovation, and Concordia University Chair Fund. AME is a
Concordia University Research Chair in Bioinorganic Chemistry. DYT is a Canada
Research Chair in Molecular Genetics. VIT is a Concordia University Research
Chair in Genomics, Cell Biology and Aging.
The authors of this manuscript have no
conflict of interests to declare.