A high glucose diet increases autophagic flux and autophagy-related genes
Similar to previous studies [16,19,20], wild type nematodes (N2 strain) exposed to a high glucose diet exhibited a significant decrease in their mean and maximal lifespans compared to controls (Figure 1A). Autophagy is considered a cellular process that maintains metabolic homeostasis when organisms are exposed to various stresses. In fact, autophagy in C. elegans is primarily considered a pro-survival mechanism [21]. Therefore, we hypothesized that high glucose could negatively affect autophagy. To assess this hypothesis, we first measured the effect of a high glucose diet on the autophagic process by measuring the expression of several ATGs. Unexpectedly, N2 animals fed 100 mM glucose had increased mRNA levels of lgg-1, pgp-2, lmp-1, and unc-51 (Figure 1B), suggesting that the autophagic process is transcriptionally active under a high glucose diet. To verify whether autophagic flux was also increased, we used a transgenic reporter strain that expressed the LGG-1 protein fused with a dimeric green fluorescent protein (dGFP) [22]; when the autophagic process is active, the dFP-LGG-1 construct is cleaved and consequently releases the protease-resistant mFP and hence indicates an increase in autophagic flux demonstrated by an increase in the mFP/dFP-LGG-1 ratio. As shown by Figure 1C and D, a significant increase in the mFP/dFP ratio was detected in nematodes treated with high glucose, indicating increased autophagic flux.
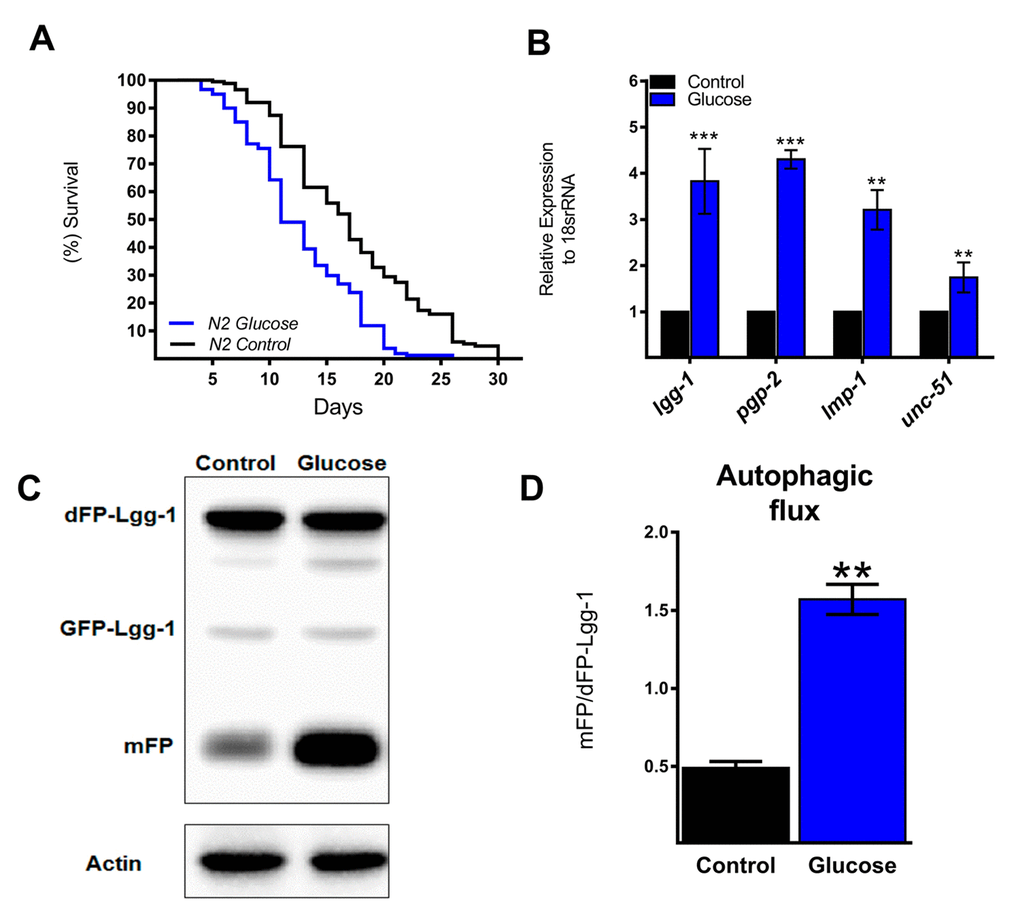
Figure 1. Autophagic flux and related genes increase with a high glucose diet. (A) Lifespan determined by Kaplan-Meier analysis of N2 wild type animals treated with a high glucose diet showed a decrease in lifespan compared to that of untreated animals. (B) Expression of selected autophagic and lysosomal genes measured by quantitative PCR (qPCR) that showed increased mRNA with high glucose. The relative expression of each gene was normalized to that of endogenous 18S rRNA. (C) Representative Western blot shows an increase in the band of mFP from the dimeric dFP-LGG-1 when worms were subjected to a high glucose diet. (D) The mFP/dFP-LGG-1 ratio indicates an increase in autophagic flux compared to that in normal conditions. *** p < 0.001; ** p < 0.01, Error bars represent ± SEM, t test with Bonferroni’s post hoc test using GraphPad Prism.
High glucose induces activation of HLH-30 and its target genes
The autophagic machinery may be transcriptionally activated by HLH-30, a C. elegans homolog of mammalian TFEB [23]. Under normal conditions, HLH-30 localizes mainly in the cytosol but rapidly translocates to the nucleus under stress conditions to promote cellular adaptation by upregulating the transcription of autophagic and lysosomal genes [23]. To investigate whether high glucose modified HLH-30 intracellular localization, we used a transgenic HLH-30::GFP reporter nematode strain fed a high glucose diet for 24 h. As shown in Figure 2A and B, a high glucose diet significantly stimulated HLH-30 nuclear localization from the cytosol to the nucleus. Consistent with its proposed autoregulatory feedback loop [11], HLH-30 mRNA increased 3-fold in the N2 strain under the same experimental conditions (Figure 2C). However, a high glucose diet had no effect on the expression of these same genes in the hlh-30 mutant strain (Figure 1B and 2D). Together, these data revealed that a high glucose diet activates ATGs through the HLH-30-activated transcription factor.
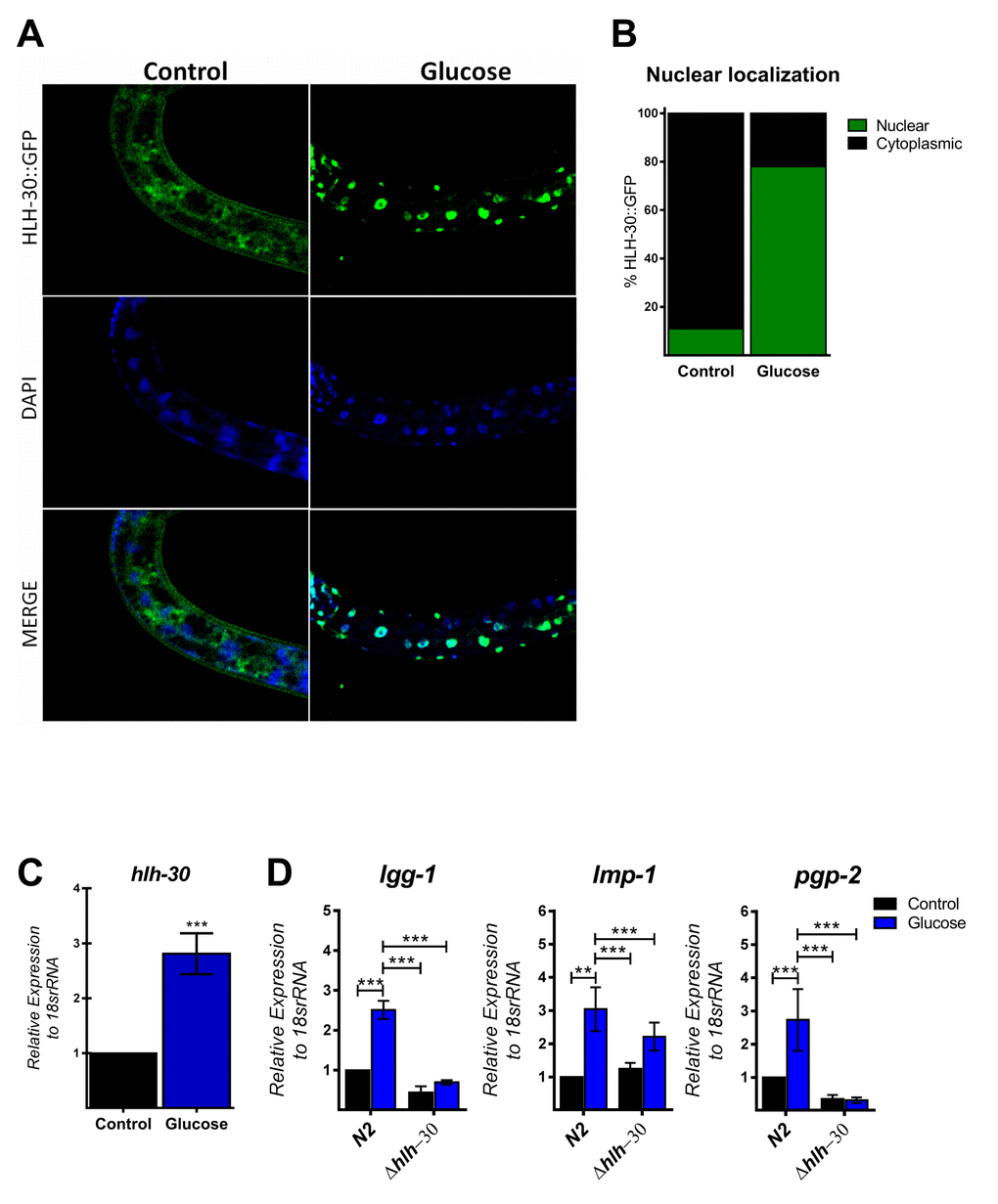
Figure 2. HLH-30 is activated by a high glucose diet. (A and B) Representative confocal images and quantitation, respectively, showing the nuclear localization of HLH-30 with the high glucose diet (green dots). Nuclei were visualized by DAPI staining (blue dots). (C) shows the expression level of hlh-30 mRNA that was increased in wild type nematodes treated with a high glucose diet. (D) mRNA expression of putative autophagy-related and lysosomal target genes in the N2 and hlh-30 (tm1978) mutant strains. Data represent the mean ± SEM of three independent experiments; *** p < 0.001, one-way ANOVA with Bonferroni’s post hoc test using GraphPad Prism.
PP1 and/or PP2A are necessary for glucose-induced HLH-30 activity in C. elegans
Because mammalian TFEB activation is achieved by dephosphorylation through calcium-dependent calcineurin protein phosphatase [24], we reasoned that a similar mechanism might be involved in HLH-30 activation. To examine whether TAX-6, an ortholog of mammalian calcineurin A, has a similar effect on HLH-30 localization, we grew the HLH-30::GFP transgenic strain under a high glucose diet and performed a tax-6 RNAi knockdown. Our results showed that tax-6 RNAi (Figure 4C) fails to inhibit the nuclear accumulation of HLH-30 in glucose-treated worms (Figure 4A and B), suggesting that HLH-30 activation is a calcineurin-independent mechanism. It has been suggested that other families of phosphatases, specifically PP2Ac, may regulate TFEB activity [15]. To investigate this hypothesis, we simultaneously treated worms with high glucose and okadaic acid (OA), a specific inhibitor of the PP1 and PP2A protein phosphatases [25], at different concentrations (30, 60, 120 and 240 nM) for 24 h and found that the nuclear localization of HLH-30 was modified in a dose-dependent manner (Supplementary Figure 2). Nematodes incubated with 30 and 60 nM of OA displayed nuclear HLH-30 with low amounts in the cytoplasm, whereas at 120 and 240 nM, the localization was preferentially cytosolic (Supplementary Figures 2A and B). Therefore, due to the high cytosolic HLH-30 detected in nematodes treated with 120 nM, we used this OA concentration, that generally is used to evaluate the effect of OA on phosphatase (PP2A) [15], to determine that the localization of HLH-30 is mediated by PP2A protein phosphatase (Figure 4A, right panel) instead of calcineurin/TAX-6 because tax-6 knockdown did not modify the cellular localization of HLH-30 (Figure 4A, middle panel).
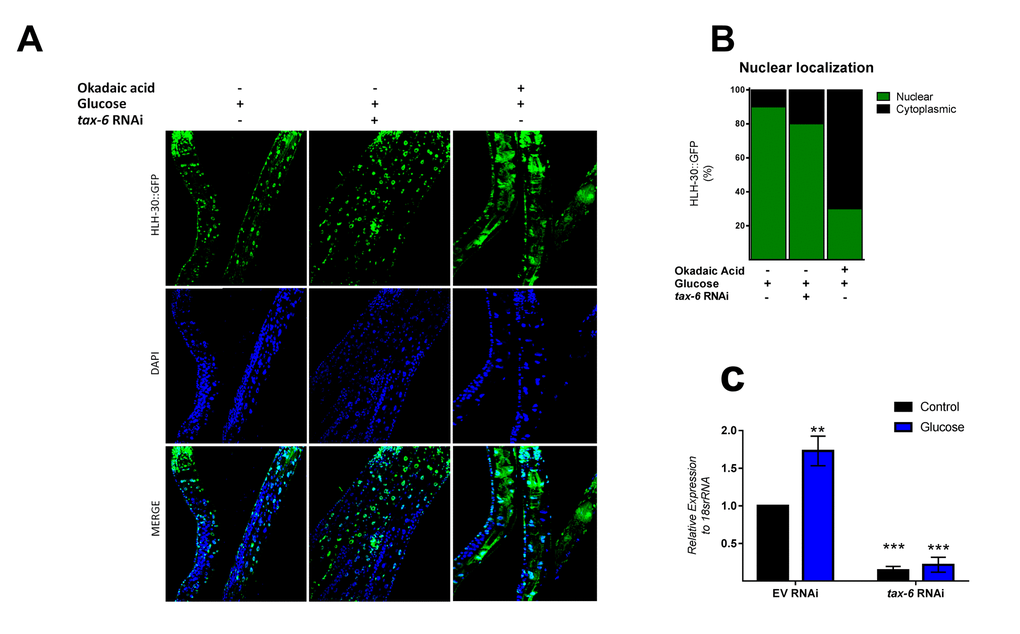
Figure 4. PPI and/or PP2A might regulate HLH-30 activation. (A) Confocal images of HLH-30::GFP worms treated with high glucose and TAX-6 interference by RNAi did not affect the nuclear localization of HLH-30, whereas pharmacologic addition of okadaic acid (120 nM) prevented it with a high glucose diet. Quantification is given in (B). Nuclei were labeled with DAPI (blue dots), (C) qRT-PCR analysis of tax-6 mRNA with or without high glucose after treatment with control (empty vector) or tax-6 RNAi. p-value (***p< 0.001, **p < 0.01). Error bars represent ± SEM, one-way ANOVA with Bonferroni’s post hoc test using GraphPad Prism.
DISCUSSION
A high glucose diet is known to decrease the lifespan of a wide range of eukaryotic organisms that include metazoans, such as the nematode C. elegans, the fruit fly Drosophila melanogaster, and various mammals [16,17,19]. However, the molecular mechanism by which high glucose levels decrease lifespan is not well understood. This report provides evidence that supports the role of the HLH-30 transcription factor in lifespan determination under a high glucose diet. We found that (i) a high glucose diet increased HLH-30 nuclear translocation and therefore its activity, which was observed by the induction at the transcriptional level of the ATGs lgg-1, lmp-1, pgp-2, and unc-51; (ii) high glucose activates autophagic flux in an HLH-30-dependent manner; and (iii) HLH-30 nuclear translocation is dependent on phosphatases unrelated to calcineurin and concomitantly enhances autophagic genes. The current study provides evidence that the activation of HLH-30-dependent autophagy is implicated in a decrease in lifespan.
TFEB/HLH-30 belongs to the MIT family of bHLH transcription factors and is considered the main regulator of autophagy and lysosomal function [7,26]. Previous studies have shown that TFEB/HLH-30 localizes predominantly in the cytoplasm in basal conditions and translocates into the nucleus upon several cellular stresses, such as lysosomal impairment, bacterial infection, prolonged ER stress, and nutrient scarcity [10–12,27]. In fact, the activation of TFEB/HLH-30 during nutrient deprivation leads to an increase in the lifespan of C. elegans; however, recent reports have shown that TFEB could respond in an unexpected manner depending on the type of stimulus [12,23]. Thus, depending on the cellular context, HLH-30 might lead to cell death, suggesting that TFEB/HLH-30 has a new role as a potent inducer of cell death in different stress conditions that have not been studied extensively thus far. Consistent with this idea, a positive relationship between the progressive nuclear translocation of TFEB and the subsequent augmentation in the expression of autophagy and lysosomal genes with high sucrose concentrations has been reported previously in HeLa cells [9,28]. Nevertheless, whether sucrose-dependent TFEB activation has deleterious effects on cellular viability has not been studied, and little is known about the mechanism involved in TFEB nuclear translocation under this stress condition.
Given the different roles of TFEB, we hypothesized that HLH-30 also affects the lifespan under a high glucose diet. Interestingly, we found that with a high glucose diet, HLH-30 increases its nuclear location and transcriptional activity, which correlates with the reduced lifespan of C. elegans, since the loss of HLH-30 resulted in partial rescue of lifespan, supporting the notion that HLH-30 activated under high glucose conditions reduces lifespan. This finding is consistent with the fact that activation of mammalian TFEB also promotes cell death, suggesting a conserved role of HLH-30 in C. elegans since its activation by glucose decreased the lifespan.
Several lines of evidence suggest that in mammals, calcineurin (PP2B), a serine/threonine protein phosphatase regulated by cellular calcium, activates several transcription factors through dephosphorylation events [24,29,30]. In mammals, calcineurin dephosphorylates and hence activates TFEB, promoting several pathways, such as autophagy, fatty acid oxidation, and immune response, among others. In C. elegans, it has been suggested that calcineurin (TAX-6) regulates lifespan through autophagy [31]; however, the mechanism of regulation of TAX-6 has not been identified. Given these findings, we reasoned that TAX-6 might regulate HLH-30 function by modulating its nuclear translocation. Our data showed that nuclear accumulation of HLH-30 with a high glucose diet is TAX-6-independent since RNAi against tax-6 did not alter HLH-30 nuclear accumulation, suggesting that other phosphatases could regulate HLH-30 nuclear localization. To test this idea, we evaluated the glucose-dependent nuclear localization of HLH-30::GFP with okadaic acid, an inhibitor of the protein phosphatases PP2A and PP1, and we observed a significant dose-dependent decrease in nuclear HLH-30, suggesting that PP2A or PP1 are targets of HLH-30, and confirmed that, as in mammals, HLH-30 is regulated by dephosphorylation events. This result is in concordance with Chen L et al., who found that hormone-dependent nuclear localization of TFEB is blocked by okadaic acid and independent of calcineurin [15]. In addition, it is important to highlight that the expression of the protein phosphatase PP2A has been reported to be increased under high glucose conditions [32,33]. Taken together, our data suggest that nuclear glucose-dependent translocation of HLH-30 is regulated by dephosphorylation events dependent on the PP2A or PP1 protein phosphatases in C. elegans.
It has been shown that autophagy, a highly conserved catabolic process, plays important roles in many physiological processes, including extension of lifespan and health promotion; however, under certain circumstances, such as prolonged cellular stress, increased autophagy can lead to cell death. Nevertheless, our understanding of the dual role of autophagy in cell survival and cell death remains incomplete. Our data provide evidence that enhanced autophagy is due to a high glucose diet in worms, resulting in a decrease in lifespan. These results are consistent with the observed role of autophagy in switching from advantageous to harmful cellular effects that may contribute to decreased lifespan, supporting the idea of the dual role of autophagy in extending or decreasing the life of multicellular organisms. Thus, our data suggest that HLH-30/TFEB-mediated autophagy contributes to limiting the lifespan of C. elegans grown with a high glucose diet. These findings indicate that the autophagic process is an active mechanism in the adverse effects of metabolic diseases, such as obesity and diabetes, because of carbohydrate-rich diets.