Melatonin alleviates meiotic defects in fetal mouse oocytes induced by Di (2-ethylhexyl) phthalate in vitro
Abstract
Di (2-ethylhexyl) phthalate (DEHP), an estrogen-like compound that is a ubiquitous environmental contaminant, has been reported to adversely affect human and mammalian reproduction. Many studies have found that exposure to DEHP during pregnancy perturbs female germ cell meiosis and is detrimental to oogenesis. Previous studies have demonstrated that melatonin (MLT) is beneficial to reproductive endocrinology, oogenesis, and embryonic development as the ability to antioxidative and antiapoptotic. However, whether the meiotic defect of germ cells exposed to DEHP could be rescued by MLT is not clear. Here, we cultured 12.5 days post coitum (dpc) fetal mouse ovaries for 6 days, exposed them to 100 μM DEHP with or without 1 μM MLT in vitro.. The results showed that DEHP exposure induced the abnormal formation of DNA double-strand breaks (DSBs), and inhibited the repair of DSBs during meiotic recombination. In addition, we found defective oocytes were prone to undergo apoptosis. Notably, this defect could be remarkably ameliorated by the addition of MLT via a reduction of the levels of reactive oxygen species and an inhibition of apoptosis. In conclusion, our data revealed that MLT had a protective action against the meiotic deterioration of fetal oocytes induced by DEHP in the mouse in vitro.
Introduction
Mammalian reproduction is critical for perpetuating and diversifying the genetic information across generations [1,2], and normal germ cell development is critical for the genetic stability of a species [3]. In female, germ cells begin entry into meiosis during the fetal stage, which is fundamental to the production of viable gametes and the propagation of a sexually reproducing species [4]. During the first meiosis prophase (MPI), DNA double-strand breaks (DSBs) sever entire chromosomes and pose a severe hazard to genomic integrity because of chromosomal fragment loss or chromosomal rearrangements [5]. These DSBs can be repaired by homologous recombination (HR) dependent mechanisms, and ultimately establish links between unassociated homologs [6,7]. The regulated repair of meiotic DSBs by HR leads to the formation of crossovers which serve to maintain a strong connection between the homologous chromosomes, thus enabling the faithful segregation of homologs during MPI [8]. Notably, a recent study has reported that Di (2-ethylhexyl) phthalate (DEHP) exposure impairs MPI and DNA damage repair in female fetal mouse germ cells in vitro, and acts through multiple pathways including DNA damage and apoptosis pathways [9].
DEHP, a ubiquitous plasticizer with estrogen-like activity, is found extensively in common consumer goods including medical equipment, and building products [10]. Due to the characteristic of being noncovalently bound, it is readily leached from products and slips into the surrounding environment [11]. This widespread presence of DEHP leads to humans being exposed via multiple ways including oral ingestion, inhalation, and dermal contact [11-13]. DEHP and its metabolites have been detected in various human tissues such as blood, urine, amniotic fluid, umbilical cord blood, breast milk, and ovarian follicular fluid [14,15]. Several studies have demonstrated that DEHP acts as an endocrine-disrupting chemical (EDC) and has adverse effects on the female reproductive system via estrogen receptors (ERs) [16,17]. Robust evidences have shown that prenatal exposure to DEHP decreased litter sizes and body weight, disrupted sex determination, caused precocious puberty and reduced circulating estradiol levels in females [18,19]. In particular, DEHP exposure in pregnant mice impairs the offspring’s ovarian development including primordial follicle assembly, follicular development and oocyte maturation [20,21]. DEHP exposure also lead to increased levels of reactive oxygen species (ROS) during follicle growth which may be responsible for the poor oocyte quality observed [22]. Furthermore, the MPI of female fetal germ cells was delayed when either the fetus or isolated female germ cells were exposed to DEHP in vitro [9,23]. Moreover, germ cell cyst breakdown was also disturbed via the adverse effect of DEHP on gap junctions [9]. Alarmingly, exposure to DEHP altered the DNA methylation pattern of imprinted genes, suggesting that DEHP exposure may lead to heritable impairment of ovarian development [23].
The amine hormone melatonin (N- Acetyl- 5- methoxy tryptamine, MLT) is synthesized and released from the pineal gland and plays an important role in the female reproductive system [24,25]. As a multifunctional molecule, MLT and its derivatives exert various biological activities including having antioxidative and anti-apoptotic effects [26,27]. Additionally, it can directly scavenge ROS and upregulate the gene expression of several antioxidant enzymes [28,29]. Notably, MLT plays a role in regulating reproductive endocrinology, oogenesis, and embryonic development [30,31]. In fact, it has been found beneficial to embryonic development being partially attributed to its ability to decrease the expression of pro-apoptotic genes and increase the level of anti-apoptotic genes, as well as reduce ROS [30,32]. Interestingly, a recent study found that DEHP adversely affected prepuberal spermato-genesis and perturbed crucial epigenetic activities in male germ cells, and that MLT was able to prevent this damage [33]. In addition, Sun et al. found EDCs decreased oocyte quality and MLT improved oocyte maturation through its rescue effects on oocyte oxidative stress mediated apoptosis and autophagy [34].
Previous studies have reported the potential toxic effects of DEHP and the protective effects of MLT on the reproductive system, no reports have examined whether MLT can improve meiotic defect of fetal mouse oocytes induced by DEHP in vitro. Therefore, in this study, we focused on the effects of MLT on improving the meiotic defects of fetal mouse oocytes exposed to DEHP.
Results
MLT relieves the abnormity of meiotic DSBs in DEHP-exposed fetal oocytes
Previously, we found that DEHP exposure during gestation perturbs female germ cell meiosis and primordial follicle assembly [9,20]. To evaluate whether DEHP exposure induces meiotic defects in fetal oocytes, 12.5 dpc fetal mouse ovaries were cultured and exposed to 100 μM DEHP for 6 days. There were no morphologically alterations in ovaries exposed to DEHP and control groups (Figure 1A). However, the protein levels of the meiosis-related synaptonemal complex protein (Sycp3) and phosphorylated H2afx protein (γH2afx, marker of DSBs) were decreased and increased respectively in the DEHP treated group (Figure 1B; P < 0.05 or P < 0.01). DEHP exposure reduced the mRNA expressions of Sycp3, but markedly increased the mRNA levels of the DNA damage-related gene Trp53 when compared with that of the control group (Figure 1C; P < 0.05 or P < 0.01).
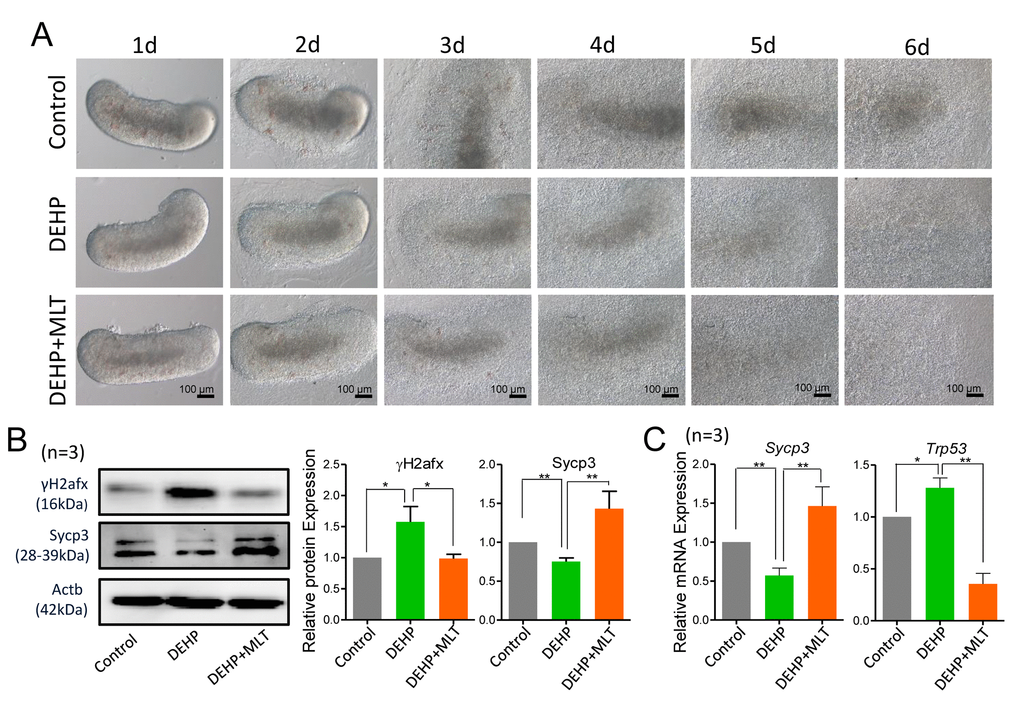
Figure 1. Effects of MLT on meiotic progression and DSBs in DEHP-exposed fetal ovaries. (A) Morphology of 12.5 dpc ovaries cultured for 6 days in control, DEHP and DEHP+MLT group in vitro. (B) Western blot analyses of the expression of Sycp3 and γH2afx protein in control, DEHP, and DEHP+MLT groups. (C) Relative expression level of genes Sycp3 and Trp53 in control, DEHP and DEHP+MLT groups. The results were presented as mean ± SEM. *P < 0.05, ** P < 0.01.
To examine whether MLT can rescue the DEHP-induced meiotic defect, based on our previous study, we treated DEHP-exposed fetal mouse ovaries with a dose of 1 μM MLT [35]. Expectedly, MLT significantly increased the level of Sycp3 and reduced the level of γH2afx protein, consistent with the mRNA levels of Sycp3 and Trp53 compared with that of DEHP treatment group (Figures 1B and 1C; P < 0.05 or P < 0.01). Similarly, there were no morphological differences between the DEHP+MLT group and the DEHP group (Figure 1A).
To further prove the reliability of the results, we double stained oocyte cytospreads for Sycp3 and γH2afx (Figure 2A). The expression pattern of γH2afx in oocytes could be classified into three main categories: none (negative or rare barely detectable foci), weaker (a few patches and less than 50% area) and stronger (numerous small and large patches, more than 50% area) [36,37]. Statistical analysis at all MPI stages indicated that the percentage of oocytes with a stronger staining pattern of γH2afx significantly increased in the DEHP treated group (87.24 ± 4.02%) compared with that of the control group (77.60 ± 5.04%) (P < 0.05), but that was much lower in the DEHP+MLT treated group (56.03 ± 9.52%) (Figure 2B; P < 0.01). Since most oocytes were in the pachytene and diplotene at 18.5 dpc, for purpose of studying the expression of γH2afx in oocytes, we further separately classified them into three categories. Similarly, the percentage of stronger γH2afx oocyte in DEHP + MLT group (64.36 ± 2.39%) is closer to control group (62.13 ± 3.37%), and much higher in DEHP group (80.48 ± 1.53%) (Figure 2C; P < 0.01).
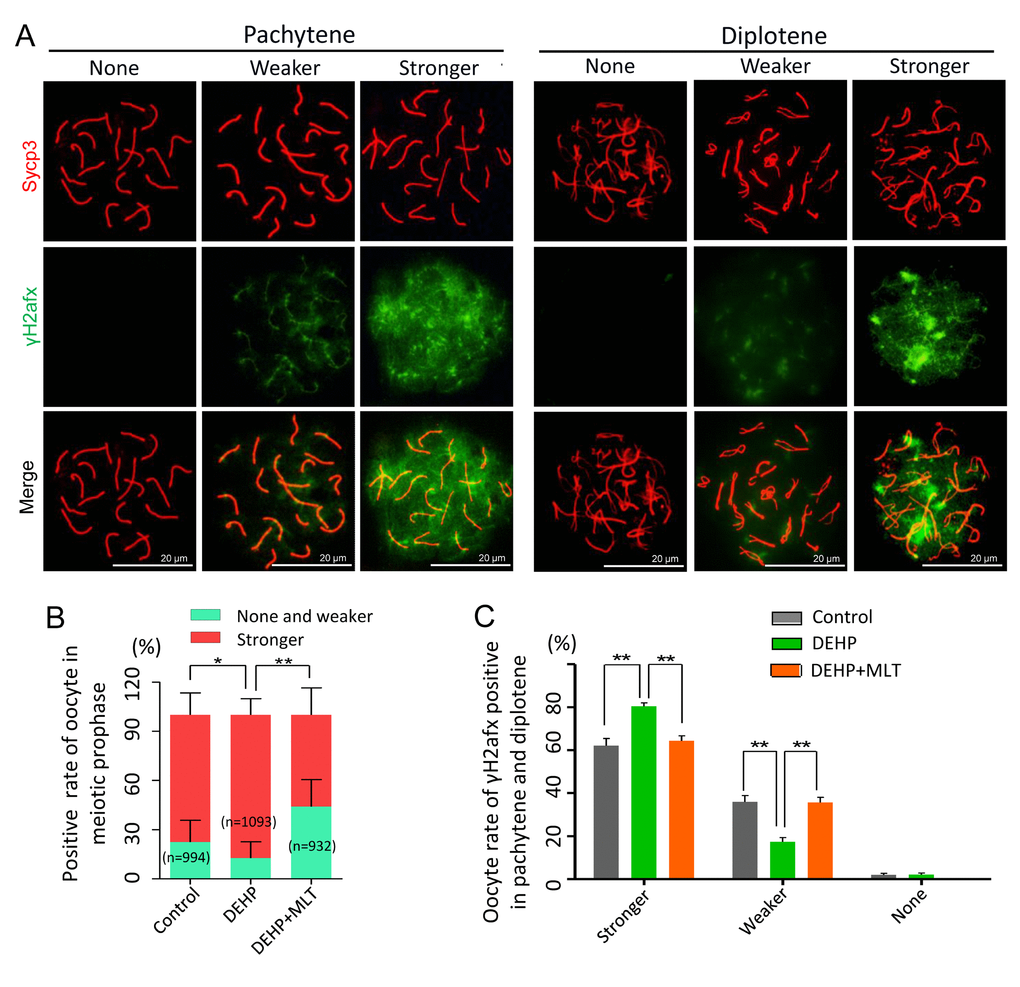
Figure 2. The effect of MLT on the formation of DSBs in fetal oocytes. (A)The immunofluorescence with Sycp3 (red) and γH2afx (green) in fetal oocytes. (B) The percentages of stronger, none and weaker γH2afx signal in oocyte in all MPI stages (control: 77.60 ± 5.04%, 22.40 ± 5.04%; DEHP: 87.24 ± 4.02%, 12.46 ± 4.02%; DEHP+MLT: 56.03 ± 9.52%, 43.97 ± 9.52%). (C) The percentages of stronger, weaker and none staining of γH2afx in pachytene and diplotene oocytes (control: 62.13 ± 3.37%, 35.92 ± 2.99%, 1.95 ± 0.78%; DEHP: 80.48 ± 1.53%, 17.41 ± 1.94%, 2.11 ± 0.78%; DEHP+MLT: 64.36 ± 2.39%, 35.64 ± 2.39%, 0.00 ± 0.00%). The results were presented as mean ± SEM. *P < 0.05, ** P < 0.01.
MLT restores the ability of DSBs repair induced by DEHP in fetal oocytes
As the repair of meiotic DSBs is crucial for HR and crossover formation, we then performed chromosome staining for the proteins RAD51 and Sycp3 (Figure 3A). The RAD51 staining pattern of meiotic oocytes was classified into three categories: none (negative), less (numbers of RAD51-positive foci ≤ 10 in each oocyte) and more (numbers of RAD51-positive foci > 10 in each oocyte) according to previous studies [38,39]. The statistical results at all MPI stages showed the percentage of positive-cells with more RAD51 signal in the DEHP group (59.83 ± 7.44%) was significantly higher than that of the control group (32.48 ± 2.54%) (Figure 3B; P < 0.01). Meanwhile, compared with the DEHP treated group, the proportions of RAD51 positive-cells in the DEHP+MLT group (33.13 ± 2.79%) was decreased (Figure 3B; P < 0.05). Likewise, there were not significant differences between the control (36.27 ± 8.02%) and DEHP + MLT (32.97 ± 4.27%) group in terms of the percentage of positive oocytes with more RAD51 signal at pachytene and diplotene stage, but the rate was increased in DEHP group (44.24 ± 2.98%) (Figure 3C).
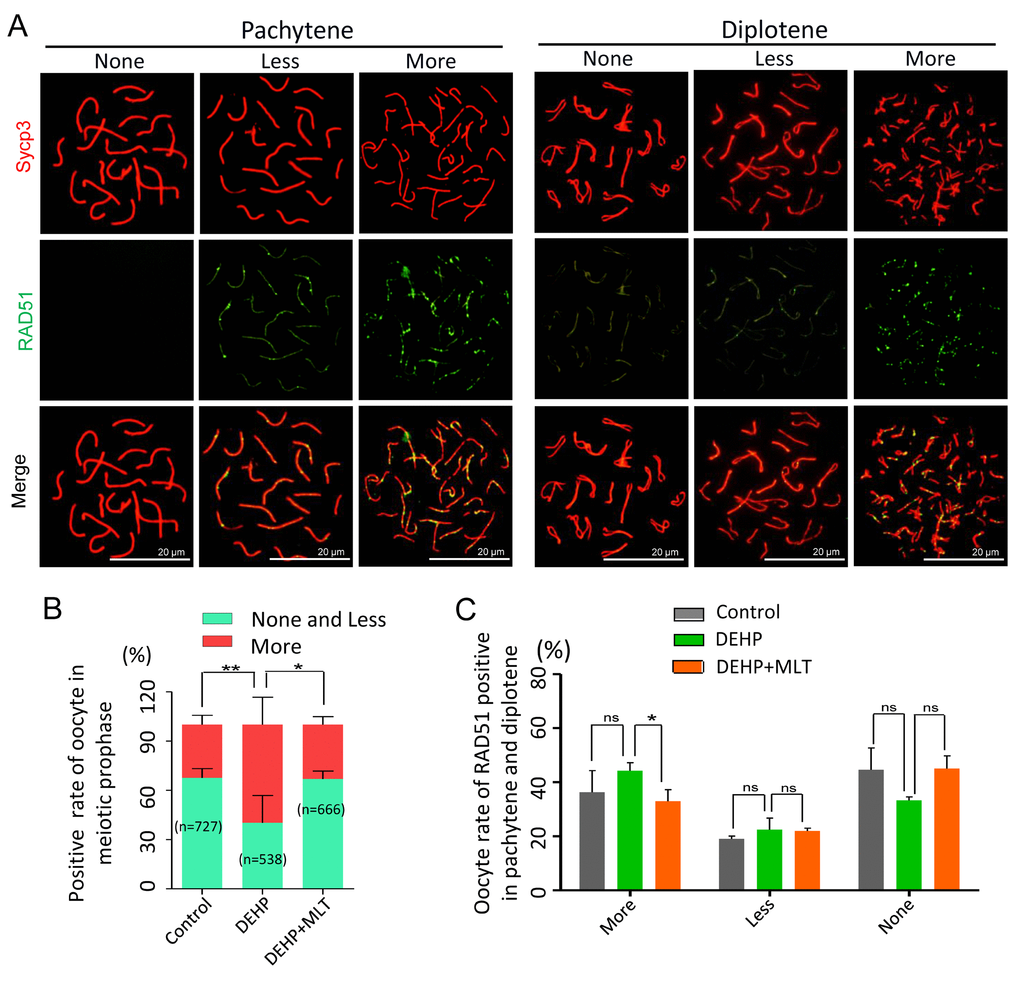
Figure 3. Effects of MLT on HR in DEHP-exposed fetal oocytes. (A)The immunofluorescence with Sycp3 (red) and RAD51 (green) in fetal oocytes. (B) The percentages of more, less and none staining of RAD51 in all MPI stages (control: 32.48 ± 2.54%, 67.52 ± 2.54%; DEHP: 59.83 ± 7.44%, 40.17 ± 7.44%; DEHP+MLT: 33.13 ± 2.79%, 66.87 ± 2.79%). (C) The percentages of more, less and none staining of RAD51 in pachytene and diplotene oocytes (control: 36.27 ± 8.02%, 19.09 ± 1.03%, 44.64 ± 8.08%; DEHP: 44.24 ± 2.98%, 22.50 ± 4.28%, 33.26 ± 1.30%; DEHP+MLT: 32.97 ± 4.27%, 22.00 ± 1.03%, 45.03 ± 4.76%). The results were presented as mean ± SEM. *P < 0.05, ** P < 0.01.
MLT improves the defect of mismatch repair caused by DEHP in fetal oocytes
Generally, successful mismatch repair is a conserved DNA repair pathway and plays a crucial role in meiotic crossover and DNA recombination. MLH1 facilitates both mismatch repair and crossover during meiosis [40]. To verify the effect of MLT on mismatch repair in the germ cells following DEHP exposure, MLH1 staining was carried out to identify the sites of exchange (Figure 4A). Based on the results of the statistical analysis, DEHP exposure significantly increased the rate of MLH1 positive oocytes (77.01 ± 4.41% vs 23.63 ± 0.55%, P < 0.05), and this increase had no significant improvement when MLT was added with DEHP (55.04 ± 17.04%) (Figure 4B). The average number of MLH1 foci in per cell showed the MLH1 foci number of oocytes at pachytene with DEHP exposure dramatically increased (13.97 ± 0.95) when compared to the control group (7.91 ± 1.33) and deceased in the presence of MLT (6.74 ± 0.58) (Figure 4C; P < 0.01). However, after MLT-administrated, the average number of MLH1 foci in per cell at diplotene (7.58 ± 1.07) was significantly decreased when compared to the DEHP treated group (13.18 ± 1.16) (P < 0.05), although there were not significant changed in control group (12.11 ± 1.74) compared to the DEHP group (Figure 4D). Collectively, these data indicated that DEHP exposure of fetal oocytes would affect the expression of MLH1, which might be one of the critical factors leading to the failure of mismatch repair. As expected, MLT alleviates mismatch repair abnormalities by influencing the level of MLH1.
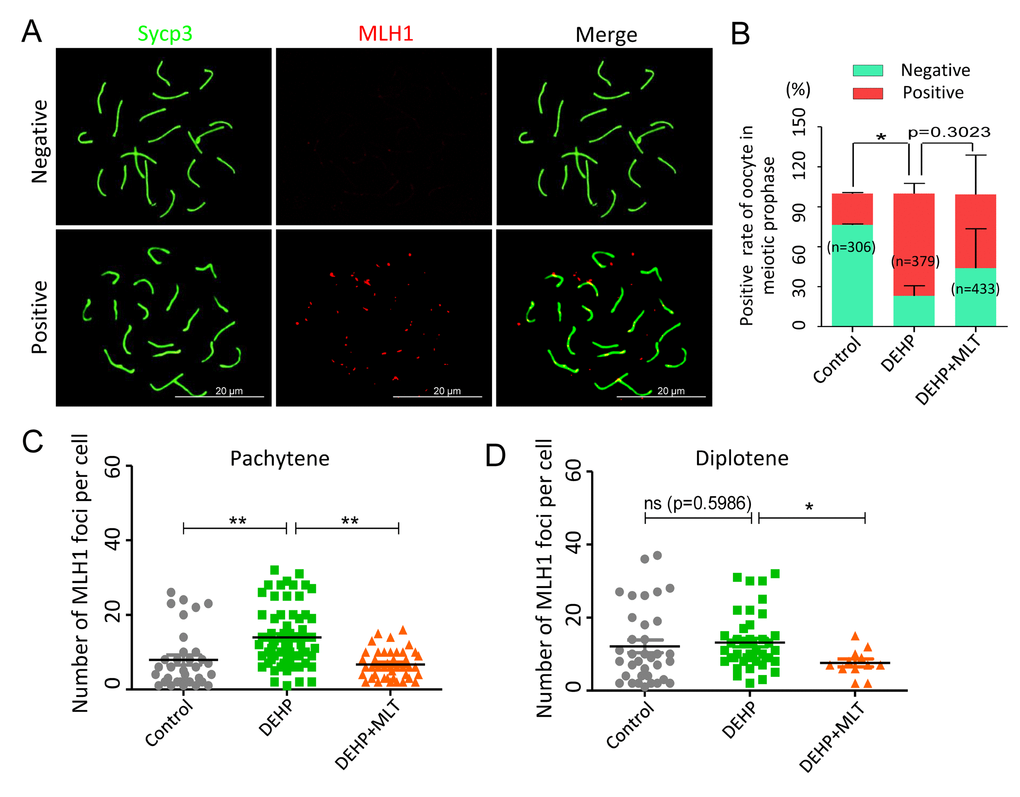
Figure 4. Effects of MLT on mismatch repair in DEHP-exposed fetal oocytes. (A)The immunofluorescence with Sycp3 (green) and MLH1 (red) in fetal oocytes. (B) The percentages of positive and negative MLH1 signal in oocytes (control: 23.63 ± 0.55%, 76.37 ± 0.55%; DEHP: 77.01 ± 4.41%, 22.99 ± 4.41%; DEHP+MLT: 55.04 ± 17.04%, 43.87 ± 17.08%). (C and D) The amounts of the MLH1 positive foci in pachytene (control: 7.91 ± 1.33; DEHP: 13.97 ± 0.95; DEHP+MLT: 6.74 ± 0.58) and diplotene (control: 12.11 ± 1.74; DEHP: 13.18 ± 1.16; DEHP+MLT: 7.58 ± 1.07) oocytes, respectively. The results were presented as mean ± SEM. *P < 0.05, ** P < 0.01.
MLT decrease ROS levels and suppresses apoptosis in DEHP-exposed fetal ovaries
Based on previous studies [34,41], we hypothesized that DEHP exposure would induce oxidative stress which would be accompanied by apoptosis in fetal oocytes. This would lead to the deterioration of critical regulators and events during oocyte meiosis. To confirm this assumption, we assessed the level of ROS in the control, DEHP, and DEHP+MLT groups (Figure 5A). In the DEHP treatment group, the fluorescent intensity of ROS was significantly increased compared to that of the control group (P < 0.01), while following the administration of MLT, ROS generation reduced dramatically (Figure 5B; P < 0.05).
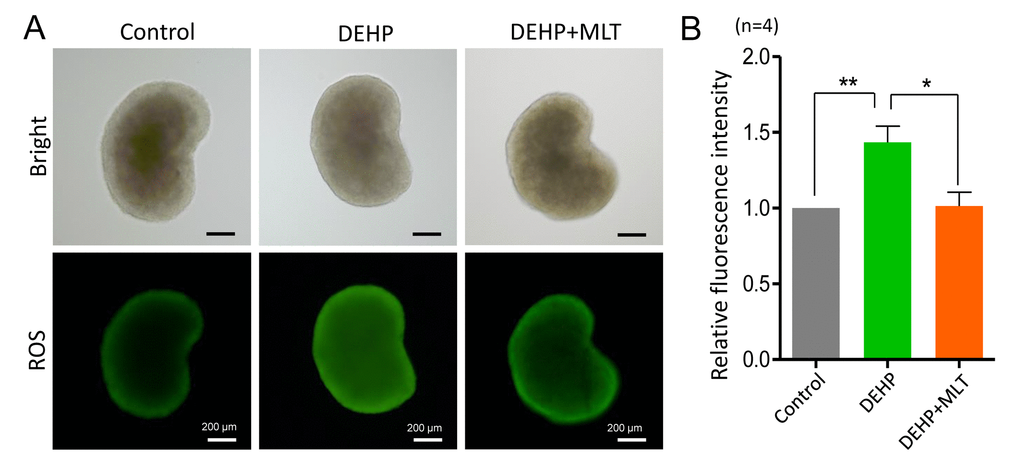
Figure 5. Effects of MLT on ROS generation in the fetal ovaries exposure to DEHP. (A) Representative images of DCHF-DA fluorescence (green) in the ovaries from the control, DEHP and DEHP+MLT groups. (B) Fluorescence intensity of ROS level. The results were presented as mean ± SEM. *P < 0.05, ** P < 0.01.
Next, we detected the apoptotic signals by TUNEL-staining in different groups. The results showed that apoptosis was hardly detected in control ovaries, but clearly existed in the DEHP-exposed group (Figure 6A). The number of TUNEL-positive cells was dramatically higher in the DEHP group when compared with that of the control group (P < 0.05) but was reduced in the DEHP+MLT group (Figure 6B; P < 0.05). Additionally, we further evaluated whether MLT influenced the expression levels of apoptotic proteins and genes, including BAX and the anti-apoptotic protein BCL-2. As expected, the ratios of BAX/BCL-2 proteins were significantly increased in the DEHP treated group compared with that of the control group (Figure 6C, P < 0.05), however, the administration of MLT restored the protein levels (P < 0.05). This observation was consistent with the gene levels (Figure 6D; P < 0.05).
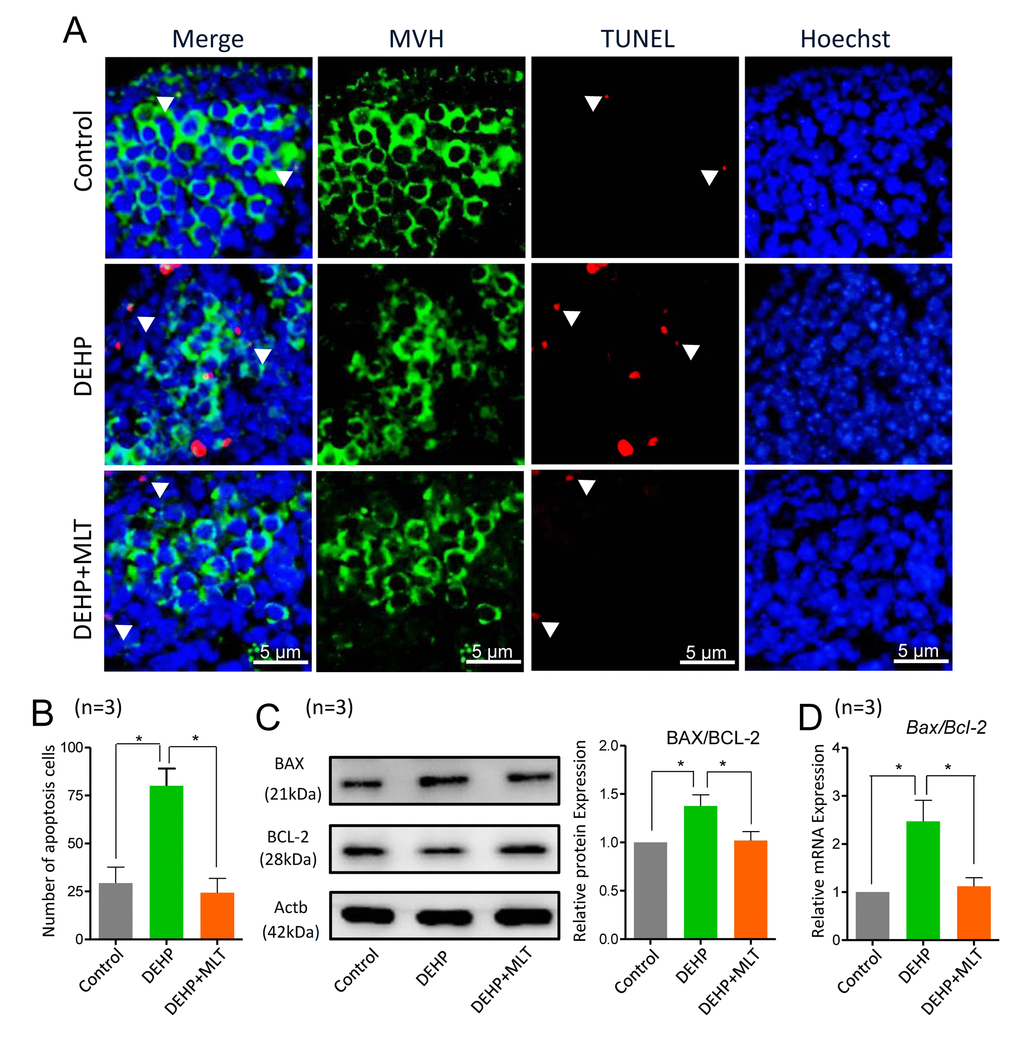
Figure 6. Effects of MLT on apoptosis in DEHP-exposed fetal ovaries. (A) Representative images of TUNEL-staining in control, DEHP, and DEHP+MLT groups. (B) The number of apoptosis positive cells in the different groups. (C) The protein expression of BAX and BCL-2 by western blot in the control, DEHP and DEHP+MLT groups. (D) The relative expression of Bax and Bcl-2 genes in the control, DEHP and DEHP+MLT groups. The results were presented as mean ± SEM. *P < 0.05.
Discussion
There is ample evidence showing that DEHP adversely impacts the mammalian reproductive system [15,42] when exposure occurs during pregnancy. As DEHP impairs fetal ovary development by impairing oocyte meiosis, this study was conducted to determine whether the meiotic impairment of female fetal germ cells induced by DEHP could be improved by MLT. In the present study, we clarified the toxic effects and possible mechanisms of DEHP exposure on fetal oocyte meiosis and confirmed the action of MLT on ameliorating DEHP-induced meiosis defects. To test it, we examined the MPI, DSBs and the ability to repair DSBs on fetal oocytes following DEHP exposure in mice. DSBs sever entire chromosomes and pose a major threat to genome integrity with unpaired DSBs leading to cell death. In this study, the results demonstrated that MLT treatment decreased the number of γH2afx-positive cells in ovaries when compared with DEHP treated. This suggests MLT plays a key role in restoring DSB induced by DEHP. Moreover, HR and crossover during MPI are required for normal meiosis in mice, and its absence leads to abnormal meiotic division and sterility in both sexes. Our finding further showed that both the expressions of RAD51 and MLH1 were lower in oocytes from ovaries treated with MLT when compared to DEHP treated. This suggests that MLT could, at least partially, restore the defects related to DSBs repair caused by DEHP exposure. Collectively, in agreement with previous studies [9], DEHP exposure inhibited the MPI progression of fetal oocytes via inducing DSBs and damaging the ability to repair DSBs. As expected, MLT treatment substantially restored DSB repair, suggesting that MLT indeed has the potential to improve the quality of fetal oocytes exposed to DEHP.
We then explored the underlying mechanisms resulting in improvement from MLT in DEHP treated fetal ovaries. Increasing studies have shown that mammalian gametes and embryos are particularly vulnerable to oxidative stress due to their plasma membrane composition [43,44]. In the present study, we observed higher oxidative stress in DEHP-exposed fetal mouse ovaries, and lower in the MLT-treated ones compared to untreated controls. Importantly, the production of ROS is accompanied with apoptosis triggering ovarian somatic cell apoptosis and leading to apoptosis of the supported oocytes [45]. Our study is consistent with previous reports that DEHP exposure induces apoptosis in fetal ovaries [9], we also showed MLT administration rescued the toxic effects of DEHP on cellular apoptosis in exposed fetal ovaries. Thus, we believe MLT as a powerful antioxidant and anti-apoptotic agent which can scavenge toxic oxygen derivatives and reduce the formation of ROS and further prevent apoptosis in fetal ovaries. Moreover, the participation of H3K9me2 induced with MLT treatment seemed to be indispensable for the rescue activity of DEHP damage [33]. In mammalians, it has been established that DNA methylation and histone modification dynamics is vital during embryonic development. As reported, DEHP exposure could affect the DNA methylation of imprinting genes in fetal mouse germ cells [46]. Also, in the present research, the meiotic DSBs and recombination of oocyte with DEHP exposure were impaired, which implied the alteration of epigenetic modification with MLT might be another potential mechanism for the rescue, it still needs further confirmation.
Interestingly, MLT and the enzymes required for its synthesis were discovered in the human placenta [47]. What’s more, studies have shown that MLT is rapidly transferred from the mother to the fetus through the placenta, and the current opinion supports that fetal MLT levels are similar to those of the mother [48-50]. In fact, MLT in the placenta is potentially associated with preeclampsia, during severe preeclampsia, placental MLT levels as well as its receptors are depressed [51]. Preeclampsia is a serious disorder with ROS production being elevated [44], thus, as a potent antioxidant MLT may be beneficial to treat preeclampsia. These findings suggest MLT may be helpful in protecting fetal oogenesis in pregnant mothers exposed to DEHP.
Conclusion
In conclusion, our results indicate that DEHP is toxic to the meiotic progress of fetal oocytes, showing increased DSBs formation and decreased DSBs repair. We further investigated oxidative stress and apoptosis as potential mechanisms for defects in fetal ovaries exposed to DEHP, and MLT is a promising agent for preventing DEHP induced toxicity.
Materials and Methods
Animal bread and ethics statement
All animals used in this study were CD-1 mice purchased from Qingdao Dacheng Furen Co. LTD (Qingdao, China). Mice were housed in temperature and light controlled environments (21 - 22 °C; 12 hours light/12 hours dark cycle). Females were mated with males at 17:00 pm and the presence of a vaginal plug was checked the next morning at 8:00 am, when detected the day was considered 0.5 dpc. All procedures described in this study were reviewed and approved by the Ethical Committee of Shenzhen Hospital of Peking University.
Ovarian culture
12.5 dpc fetal mouse ovaries were isolated and cultured as previously described [52]. Briefly, fetal mouse ovaries were dissected in half from 12.5 dpc embryos and cultured in 600 µL culture medium, consisting of α-minimal essential medium (α-MEM; Hyclone, SH30265.01B, Beijing, China), 10% fetal bovine serum (FBS; Gibco, 10099-141, USA), 0.23 mM sodium pyruvate (Hyclone, SH40003-12), 100 IU/ml of penicillin G, and 100 mg/ml of streptomycin sulfate, 10 mIU/ml follicle stimulating hormone (FSH; RD, 5925-FS, USA). Ovaries were cultured in humidified incubator at 37 °C, 5% CO2 in air and medium was changed every other day. At day six, the ovaries were collected and kept for further analysis. In vitro cultured ovaries were exposed to DEHP at a dose of 100 μM and 1 μM MLT, 0.1% DMSO and 0.1% alcohol were added to the culture as a vehicle control.
DEHP was obtained from Sigma-Aldrich (36735-1G, Saint Louis, MO, USA). Based on our previous study [9], DEHP was prepared at the concentrations of 0.254 M and 2.54 M in dimethylsulfoxide (DMSO) and the final concentration in the culture medium was 100 μM. Melatonin (M5250-1G) was purchased from Sigma-Aldrich and dissolved in 100% ethanol, which was added to the culture medium at the final concentration of 1 μM.
Oocyte cytospreads
Staining of meiotic chromosomes were evaluated using oocyte cytospreads as previous study [53]. Ovaries were collected and incubated in hypo extraction buffer for 1.5 hours, fixed with 1% PFA overnight at room temperature. Fixed samples were blocked with antibody dilution buffer (ADB) at 37 °C for half an hour and incubated with primary antibodies (Table S1) at 37 °C for 8 hours. The slides were further blocked with ADB overnight at 4 °C. The next day, the slides were incubated with secondary antibodies of CY3 (anti-rabbit, Beyotime, A0516, Nantong, China; anti-mouse, Beyotime, A0568) or FITC- labeled goat IgG (anti-mouse, Beyotime, A0521; anti-rabbit, Beyotime, A0516) at 37 °C for 1.5 hours in the dark. Hoechst 33342 (Beyotime, C1022) was used to stain nuclei for 5 min and slides were mounted with Vectashield (Vector, H-1000, Shanghai, China). Finally, slides were analyzed under a fluorescence microscope (Olympus BX51, Japan). Experiments were repeated at least 3 to 4 times and every independent experiment for oocyte counting was at least 250.
According to the characteristic morphologies of chromosomes after cytospread staining with a Sycp3 antibody, the meiotic progression was clarified into leptotene, zygotene, pachytene, and diplotene. In the leptotene, chromosomes are decondensed and long. At zygotene, chromosomes become increasingly longer and formed the synaptonemal complex (SC). During pachytene, chromosomes are short and condensed, and homologs are fully synapsis. Entering diplotene, chiasmata are formed during homologous and the SC starts to disassemble [54].
ROS level assay
To determine the levels of intracellular ROS production, fetal mouse ovaries were collected after culturing for 6 days in vitro and were then washed three times with phosphate buffer saline (PBS). Next, ovaries were incubated with the oxidation-sensitive florescent probe dichlorodihydrofluorescein (DCFH) for 30 min at 37 °C in D-PBS that contained 10 μM DCFH diacetate (DCFH-DA) (Beyotime, E004). After washing three times in D-PBS containing 0.1% BSA, ovaries were placed on glass slides, and the florescence intensity of each ovary was measured with a fluorescent microscope (Olympus BX51, Japan). For fluorescence intensity, the photographs of each independent experiment were taken under microscope with the same parameter settings (exposure time and intensity). The quantify of fluorescence intensity was examined with the ImageJ software, with the option of Plugins, the RGB measure of Analyze item was applied, the green channel then represented the fluorescence intensity of ROS.
TUNEL staining
Bright Red Apoptosis Detect Kit (Vazyme, A113-02, China) was used for the detection of TUNEL positive cells as previously described [55]. Briefly, paraffin sections were heated at 60 °C for 2 hours, washed in xylene and rehydrated through a series of ethanol gradations. After antigen retrieval at 96 °C, sections were blocked and incubated with anti-MVH protein at 4 °C overnight, then FITC-labeled goat anti-rabbit IgG was used as a secondary antibody. Washed three times before sections were treated with proteinase K for 10 min, then according to the manufactures’ instructions, carried on an incubation with the TUNEL treatment mixture at 37 °C for an hour. Finally, nuclei were counterstained with Hoechst 33342. Images were taken under a fluorescence microscope (Olympus, BX51).
Western blot
Proteins were extracts from ovaries using Cell Lysis Buffer (Beyotime, P0013) for western blot analysis according to standard methods [56]. The proteins were separated on a 10% SDS-PAGE gel and transferred onto polyvinylidene fluoride membrane (PVDF, immobilon-PSQ transfer membranes, Millipore, ISEQ00010, USA). The membrane was blocked with TBST buffer (tris buffered saline, with tween-20) containing 5 - 10% BSA and incubated in primary antibody (Table S1) overnight at 4 °C. After three washes in TBST, the membrane was incubated with HRP conjugated goat anti-rabbit or anti-mouse IgG (Beyotime, A0216) diluted in TBST at room temperature for 1.5 hours. Finally, the membranes were reacted with BeyoECL Plus Kit (Beyotime, P0018). IPWIN software was used for density measurements.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from ovaries using the RNA prep pure Micro Kit (Aidlab, RN28, Beijing, China) according to the manufacturer’s instructions. Then, the RNA was reverse-transcribed into cDNA using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech AT311-03, Beijing, China). All primers used in this research are listed in Supplementary Table S2. Relative quantification analysis was carried out with a LightCycler 480 II (Roche) using SYBR® Premix Ex Taq™ II (TAKARA, RR820A, Dalian, China). Gene expression levels were calculated using β-actb for normalization. Relative transcript abundance was calculated using the 2-ΔΔCt method [57].
Statistical analysis
Results were obtained from at least three independent experiments and are expressed as mean ± SEM. Data were statistically analyzed with GraphPad Prism software and significant difference was determined with a Student’s unpaired t-Test of independent samples. P < 0.05 was considered as significant difference, while P < 0.01 was a highly significant difference.
Author Contributions
Dr. Sun Z.Y. and Shen W. designed the study and applied for Research Ethics Board approval. Drs Zhai Q.Y., Zhang P., Wang J.J., Liu J.C. and Li L. collected and analyzed the data and prepared draft figures and tables. Drs Zhai Q.Y. prepared the manuscript draft with important intellectual input from Drs Zhang P., Wang J.J., Liu J.C. and Li L. Drs Zhai Q.Y., Shen W. and Sun Z.Y. had complete access to the study data. All authors approved the final manuscript.
Acknowledgements
We would like to thank Prof. Paul W. Dyce for his careful editing of this manuscript.
Conflicts of Interest
The authors have no conflict of interests to declare.
Funding
This work was supported by the Fund of Shenzhen City (SZSM201612066), Chengdu Women’s & Children’s Central Hospital Foundation (1743), Chongqing Bonel Foundation and the Fund for Doctoral Scientific Research Startup of Qingdao Agricultural University (6631116019).
References
-
1.
Sarraj MA, Drummond AE. Mammalian foetal ovarian development: consequences for health and disease. Reproduction. 2012; 143:151–63. https://doi.org/10.1530/REP-11-0247 [PubMed]
-
2.
Wear HM, McPike MJ, Watanabe KH. From primordial germ cells to primordial follicles: a review and visual representation of early ovarian development in mice. J Ovarian Res. 2016; 9:36. https://doi.org/10.1186/s13048-016-0246-7 [PubMed]
-
3.
Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007; 134:3401–11. https://doi.org/10.1242/dev.001107 [PubMed]
-
4.
Spiller CM, Bowles J, Koopman P. Regulation of germ cell meiosis in the fetal ovary. Int J Dev Biol. 2012; 56:779–87. https://doi.org/10.1387/ijdb.120142pk [PubMed]
-
5.
Gospodinov A, Herceg Z. Chromatin structure in double strand break repair. DNA Repair (Amst). 2013; 12:800–10. https://doi.org/10.1016/j.dnarep.2013.07.006 [PubMed]
-
6.
Li W, Ma H. Double-stranded DNA breaks and gene functions in recombination and meiosis. Cell Res. 2006; 16:402–12. https://doi.org/10.1038/sj.cr.7310052 [PubMed]
-
7.
Page SL, Hawley RS. Chromosome choreography: the meiotic ballet. Science. 2003; 301:785–89. https://doi.org/10.1126/science.1086605 [PubMed]
-
8.
Gray S, Cohen PE. Control of Meiotic Crossovers: From Double-Strand Break Formation to Designation. Annu Rev Genet. 2016; 50:175–210. https://doi.org/10.1146/annurev-genet-120215-035111 [PubMed]
-
9.
Liu JC, Lai FN, Li L, Sun XF, Cheng SF, Ge W, Wang YF, Li L, Zhang XF, De Felici M, Dyce PW, Shen W. Di (2-ethylhexyl) phthalate exposure impairs meiotic progression and DNA damage repair in fetal mouse oocytes in vitro. Cell Death Dis. 2017; 8:e2966. https://doi.org/10.1038/cddis.2017.350 [PubMed]
-
10.
Rattan S, Brehm E, Gao L, Niermann S, Flaws JA. Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod. 2018; 98:130–45. https://doi.org/10.1093/biolre/iox154 [PubMed]
-
11.
Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007; 210:623–34. https://doi.org/10.1016/j.ijheh.2007.07.011 [PubMed]
-
12.
Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne). 2015; 6:8. https://doi.org/10.3389/fendo.2015.00008 [PubMed]
-
13.
Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci. 2009; 364:2063–78. https://doi.org/10.1098/rstb.2008.0208 [PubMed]
-
14.
Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Håkansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008; 116:334–39. [PubMed]
-
15.
Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate. 2003; 83:22–24. https://doi.org/10.1159/000067012 [PubMed]
-
16.
Marques-Pinto A, Carvalho D. Human infertility: are endocrine disruptors to blame? Endocr Connect. 2013; 2:R15–29. https://doi.org/10.1530/EC-13-0036 [PubMed]
-
17.
Lai FN, Liu JC, Li L, Ma JY, Liu XL, Liu YP, Zhang XF, Chen H, De Felici M, Dyce PW, Shen W. Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice. Arch Toxicol. 2017; 91:1279–92. https://doi.org/10.1007/s00204-016-1790-z [PubMed]
-
18.
Wang Y, Yang Q, Liu W, Yu M, Zhang Z, Cui X. DEHP exposure in utero disturbs sex determination and is potentially linked with precocious puberty in female mice. Toxicol Appl Pharmacol. 2016; 307:123–29. https://doi.org/10.1016/j.taap.2016.08.001 [PubMed]
-
19.
Martinez-Arguelles DB, Papadopoulos V. Prenatal phthalate exposure: epigenetic changes leading to lifelong impact on steroid formation. Andrology. 2016; 4:573–84. https://doi.org/10.1111/andr.12175 [PubMed]
-
20.
Zhang T, Li L, Qin XS, Zhou Y, Zhang XF, Wang LQ, De Felici M, Chen H, Qin GQ, Shen W. Di-(2-ethylhexyl) phthalate and bisphenol A exposure impairs mouse primordial follicle assembly in vitro. Environ Mol Mutagen. 2014; 55:343–53. https://doi.org/10.1002/em.21847 [PubMed]
-
21.
Zhang T, Shen W, De Felici M, Zhang XF. Di(2-ethylhexyl)phthalate: adverse effects on folliculogenesis that cannot be neglected. Environ Mol Mutagen. 2016; 57:579–88. https://doi.org/10.1002/em.22037 [PubMed]
-
22.
Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod. 2012; 87:152. https://doi.org/10.1095/biolreprod.112.102467
[PubMed]
-
23.
Zhang XF, Zhang T, Han Z, Liu JC, Liu YP, Ma JY, Li L, Shen W. Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod Fertil Dev. 2015; 27:1213–21. https://doi.org/10.1071/RD14113 [PubMed]
-
24.
Anderson G, Vaillancourt C, Maes M, Reiter RJ. Breastfeeding and the gut-brain axis: is there a role for melatonin? Biomol Concepts. 2017; 8:185–95. https://doi.org/10.1515/bmc-2017-0009 [PubMed]
-
25.
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007; 42:28–42. https://doi.org/10.1111/j.1600-079X.2006.00407.x [PubMed]
-
26.
Zhang M, Dai X, Lu Y, Miao Y, Zhou C, Cui Z, Liu H, Xiong B. Melatonin protects oocyte quality from Bisphenol A-induced deterioration in the mouse. J Pineal Res. 2017; 62:e12396. https://doi.org/10.1111/jpi.12396 [PubMed]
-
27.
Amin AH, El-Missiry MA, Othman AI. Melatonin ameliorates metabolic risk factors, modulates apoptotic proteins, and protects the rat heart against diabetes-induced apoptosis. Eur J Pharmacol. 2015; 747:166–73. https://doi.org/10.1016/j.ejphar.2014.12.002 [PubMed]
-
28.
Tian X, Wang F, Zhang L, Ji P, Wang J, Lv D, Li G, Chai M, Lian Z, Liu G. Melatonin Promotes the In Vitro Development of Microinjected Pronuclear Mouse Embryos via Its Anti-Oxidative and Anti-Apoptotic Effects. Int J Mol Sci. 2017; 18:E988. https://doi.org/10.3390/ijms18050988 [PubMed]
-
29.
Wang T, Gao YY, Chen L, Nie ZW, Cheng W, Liu X, Schatten H, Zhang X, Miao YL. Melatonin prevents postovulatory oocyte aging and promotes subsequent embryonic development in the pig. Aging (Albany NY). 2017; 9:1552–64. https://doi.org/10.18632/aging.101252 [PubMed]
-
30.
Gao C, Han HB, Tian XZ, Tan DX, Wang L, Zhou GB, Zhu SE, Liu GS. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J Pineal Res. 2012; 52:305–11. https://doi.org/10.1111/j.1600-079X.2011.00944.x [PubMed]
-
31.
Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006; 228:333–43. https://doi.org/10.1016/j.tox.2006.09.018 [PubMed]
-
32.
Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM. Melatonin and reproduction revisited. Biol Reprod. 2009; 81:445–56. https://doi.org/10.1095/biolreprod.108.075655 [PubMed]
-
33.
Zhang T, Zhou Y, Li L, Zhao Y, De Felici M, Reiter RJ, Shen W. Melatonin protects prepuberal testis from deleterious effects of bisphenol A or diethylhexyl phthalate by preserving H3K9 methylation. J Pineal Res. 2018; 65:e12497. https://doi.org/10.1111/jpi.12497 [PubMed]
-
34.
Zhang Y, Wang T, Lan M, Zang XW, Li YL, Cui XS, Kim NH, Sun SC. Melatonin protects oocytes from MEHP exposure-induced meiosis defects in porcine. Biol Reprod. 2018; 98:286–98. https://doi.org/10.1093/biolre/iox185 [PubMed]
-
35.
Wang YF, Sun XF, Han ZL, Li L, Ge W, Zhao Y, De Felici M, Shen W, Cheng SF. Protective effects of melatonin against nicotine-induced disorder of mouse early folliculogenesis. Aging (Albany NY). 2018; 10:463–80. https://doi.org/10.18632/aging.101405 [PubMed]
-
36.
Wang H, Höög C. Structural damage to meiotic chromosomes impairs DNA recombination and checkpoint control in mammalian oocytes. J Cell Biol. 2006; 173:485–95. https://doi.org/10.1083/jcb.200512077 [PubMed]
-
37.
Wang JJ, Yu XW, Wu RY, Sun XF, Cheng SF, Ge W, Liu JC, Li YP, Liu J, Zou SH, De Felici M, Shen W. Starvation during pregnancy impairs fetal oogenesis and folliculogenesis in offspring in the mouse. Cell Death Dis. 2018; 9:452. https://doi.org/10.1038/s41419-018-0492-2 [PubMed]
-
38.
Liu KH, Sun XF, Feng YZ, Cheng SF, Li B, Li YP, Shen W, Li L. The impact of Zearalenone on the meiotic progression and primordial follicle assembly during early oogenesis. Toxicol Appl Pharmacol. 2017; 329:9–17. https://doi.org/10.1016/j.taap.2017.05.024 [PubMed]
-
39.
Malki S, van der Heijden GW, O’Donnell KA, Martin SL, Bortvin A. A role for retrotransposon LINE-1 in fetal oocyte attrition in mice. Dev Cell. 2014; 29:521–33. https://doi.org/10.1016/j.devcel.2014.04.027 [PubMed]
-
40.
Santucci-Darmanin S, Walpita D, Lespinasse F, Desnuelle C, Ashley T, Paquis-Flucklinger V. MSH4 acts in conjunction with MLH1 during mammalian meiosis. FASEB J. 2000; 14:1539–47. https://doi.org/10.1096/fj.99-0851com [PubMed]
-
41.
Ambruosi B, Uranio MF, Sardanelli AM, Pocar P, Martino NA, Paternoster MS, Amati F, Dell’Aquila ME. In vitro acute exposure to DEHP affects oocyte meiotic maturation, energy and oxidative stress parameters in a large animal model. PLoS One. 2011; 6:e27452. https://doi.org/10.1371/journal.pone.0027452 [PubMed]
-
42.
Kim HY. Risk assessment of di(2-ethylhexyl) phthalate in the workplace. Environ Health Toxicol. 2016; 31:e2016011. . http://doi.org/10.5620/eht.e2016011 https://doi.org/10.5620/eht.e2016011 [PubMed]
-
43.
Khalil WA, Marei WF, Khalid M. Protective effects of antioxidants on linoleic acid-treated bovine oocytes during maturation and subsequent embryo development. Theriogenology. 2013; 80:161–68. https://doi.org/10.1016/j.theriogenology.2013.04.008 [PubMed]
-
44.
Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, Reiter RJ. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009; 92:328–43. https://doi.org/10.1016/j.fertnstert.2008.05.016 [PubMed]
-
45.
Khazaei M, Aghaz F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int J Fertil Steril. 2017; 11:63–70. https://doi.org/10.22074/ijfs.2017.4995 [PubMed]
-
46.
Li L, Zhang T, Qin XS, Ge W, Ma HG, Sun LL, Hou ZM, Chen H, Chen P, Qin GQ, Shen W, Zhang XF. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep. 2014; 41:1227–35. https://doi.org/10.1007/s11033-013-2967-7 [PubMed]
-
47.
Lanoix D, Beghdadi H, Lafond J, Vaillancourt C. Human placental trophoblasts synthesize melatonin and express its receptors. J Pineal Res. 2008; 45:50–60. https://doi.org/10.1111/j.1600-079X.2008.00555.x [PubMed]
-
48.
Korenevsky AV, Milyutina YP, Bukalyov AV, Baranova YP, Vinogradova IA, Arutjunyan AV. Protective effect of melatonin and epithalon on hypothalamic regulation of reproduction in female rats in its premature aging model and on estrous cycles in senescent animals in various lighting regimes. Adv Gerontol. 2013; 26:263–74. Protective effect of melatonin and epithalon on hypothalamic regulation of reproduction in female rats in its premature aging model and on estrous cycles in senescent animals in various lighting regimes [PubMed]
-
49.
Kennaway DJ, Goble FC, Stamp GE. Factors influencing the development of melatonin rhythmicity in humans. J Clin Endocrinol Metab. 1996; 81:1525–32. https://doi.org/10.1210/jcem.81.4.8636362 [PubMed]
-
50.
Okatani Y, Okamoto K, Hayashi K, Wakatsuki A, Tamura S, Sagara Y. Maternal-fetal transfer of melatonin in pregnant women near term. J Pineal Res. 1998; 25:129–34. https://doi.org/10.1111/j.1600-079X.1998.tb00550.x [PubMed]
-
51.
Lanoix D, Guérin P, Vaillancourt C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: new insights into the role of this hormone in pregnancy. J Pineal Res. 2012; 53:417–25. https://doi.org/10.1111/j.1600-079X.2012.01012.x [PubMed]
-
52.
Feng YM, Liang GJ, Pan B, Qin XS, Zhang XF, Chen CL, Li L, Cheng SF, De Felici M, Shen W. Notch pathway regulates female germ cell meiosis progression and early oogenesis events in fetal mouse. Cell Cycle. 2014; 13:782–91. https://doi.org/10.4161/cc.27708 [PubMed]
-
53.
Liang GJ, Zhang XF, Wang JJ, Sun YC, Sun XF, Cheng SF, Li L, De Felici M, Shen W. Activin A accelerates the progression of fetal oocytes throughout meiosis and early oogenesis in the mouse. Stem Cells Dev. 2015; 24:2455–65. https://doi.org/10.1089/scd.2015.0068 [PubMed]
-
54.
Bolcun-Filas E, Schimenti JC. Genetics of meiosis and recombination in mice. Int Rev Cell Mol Biol. 2012; 298:179–227. https://doi.org/10.1016/B978-0-12-394309-5.00005-5 [PubMed]
-
55.
Sun YC, Wang YY, Sun XF, Cheng SF, Li L, Zhao Y, Shen W, Chen H. The role of autophagy during murine primordial follicle assembly. Aging (Albany NY). 2018; 10:197–211. https://doi.org/10.18632/aging.101376 [PubMed]
-
56.
Wang YY, Sun YC, Sun XF, Cheng SF, Li B, Zhang XF, De Felici M, Shen W. Starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes. Cell Death Dis. 2017; 8:e2613. https://doi.org/10.1038/cddis.2017.3 [PubMed]
-
57.
Zhang LJ, Chen B, Feng XL, Ma HG, Sun LL, Feng YM, Liang GJ, Cheng SF, Li L, Shen W. Exposure to Brefeldin A promotes initiation of meiosis in murine female germ cells. Reprod Fertil Dev. 2015; 27:294–303. https://doi.org/10.1071/RD13281 [PubMed]