Introduction
4-Hydroxynonenal (4‑HNE) is the
product of peroxidation of n-6 polyunsaturated fatty acids [1,2]. 4‑HNE
is strongly electrophilic and thus bioactive because of its potential to modify
biological macromolecules, particularly proteins, via the formation of covalent
adducts [3,4]. These chemical properties of 4‑HNE render the compound
toxic at high concentrations [3]. However, at moderate (physiological) levels
[5], 4‑HNE acts as a messenger molecule that signals the presence of a
pro-oxidant state and elicits a variety of appropriate biological responses
(reviewed in refs. [4, 6-9]). In this context, lipid peroxidation acts as a
sensor that registers the presence of oxidants and translates it into the generation
of electrophiles such as 4‑HNE.
Compared with oxidantssuch
as most types of ROS (reactive oxygen species), 4‑HNE is chemically
better suited for the role of a signaling molecule because of its longer
half-life and thus greater range of diffusion, and a higher selectivity for
reaction with specific targets.
Multiple
physiological and pathophysiological situations can result in oxidative
stress. Among them is excessive fat accumulation that leads to mild but chronic
inflammation characterized by focal necrosis and recruitment of macrophages to
the adipose tissue; inflammation is both local and systemic [10-12]. The oxidative stress that accompanies
inflammation would be expected to trigger lipid peroxidation and 4‑HNE
production. Indeed, higher levels of 4‑HNE have been found in obese than
in non-obese humans and mice [13,14].
Several biologically fundamental target processes responsive to 4‑HNE
signaling have been identified, including modulation of apoptosis, activation
of stress response pathways, cell proliferation and differentiation,
mitochondrial coupling, and others (reviewed in refs. [9, 15-18]). Results
presented in this paper, together with our previous data [19], define a new
target of 4‑HNE signaling: adipogenesis. This would place 4‑HNE not
only downstream [14] but also upstream of fat accumulation, resulting in a
positive feedback loop. In this paper, we present evidence for a biochemical
mechanism by which 4‑HNE could trigger increased lipid storage, and
discuss possible adaptive and maladaptive roles of the self-reinforcing loop
that involves triglycerides and 4‑HNE, in particular, in the context of
aging.
Results
Expression of the gst‑10 gene product, which is capable of
conjugating 4‑HNE, is inversely related to the level of whole-body 4‑HNE-protein
adducts
As we have
previously shown [20,21], the capacity of C. elegans to conjugate
4‑HNE with glutathione was reduced by RNAi-mediated knockdown of
CeGSTP2-2, the product of the gst‑10 gene. The loss of 4‑HNE-conjugating
activity was only partial because of the existence of multiple GSTs capable of
conjugating 4‑HNE [20]. Nevertheless, silencing of gst‑10 resulted in a greater than 50% increase of the level of whole-body 4‑HNE-protein
adducts (Figure 1). Conversely, overexpression of gst‑10 results
in an enhanced capacity to conjugate 4‑HNE and in a decrease in the
content of 4‑HNE-protein adducts [22]. Because the biological effects of
4‑HNE are thought to be mediated mostly by formation of covalent adducts
with proteins, a process that frequently alters protein function (reviewed in
refs. [4, 6-9]), the observed changes in adduct amounts could be functionally
significant.
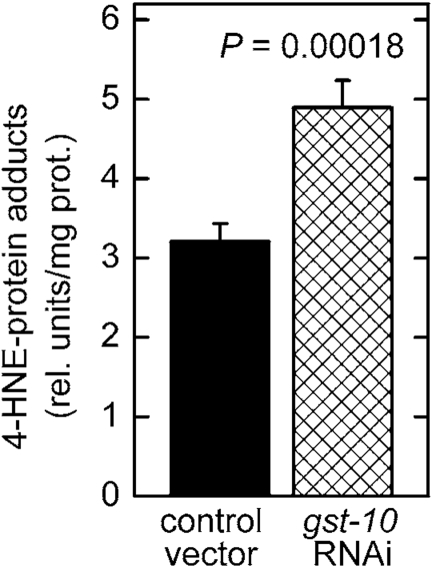
Figure 1. Increased level of 4‑HNE-protein adducts in C. elegans with silenced gst‑10 gene.
RNAi silencing of gst‑10 was carried out as previously described
[21] in C. elegans strain Bristol-N2; worms fed bacteria
transformed with an insert-free L4440 feeding vector served as the control.
The level of 4‑HNE-protein adducts was determined in 5-day-old worms
by competitive ELISA [93]. The values shown are means ± S.D. of 4
biological replicates (independently grown batches of worms) per group.
Fat accumulation in C. elegans is inversely
proportional to the expression level of gst-10
Worms subjected to RNAi against gst‑10 accumulate significant amounts of fat (Figures 2A and 2B). The use of two
independent lipid stains, Nile red [23,24] and Sudan black [25], minimizes the
likelihood of artifacts. In contrast to animals subjected to RNAi, several C. elegans lines overexpressing the gst‑10 gene product [22] had a lean
phenotype (Figure 2C). This lean phenotype was confirmed using a second,
independently derived control line carrying an insert-free vector (data not
shown), thus establishing that the low fat content of gst-10-overecxpressing
worms was not due to an aberrant control line. The data demonstrate an inverse
proportionality between gst‑10-linked 4‑HNE-conjugating
activity and fat accumulation in C. elegans.
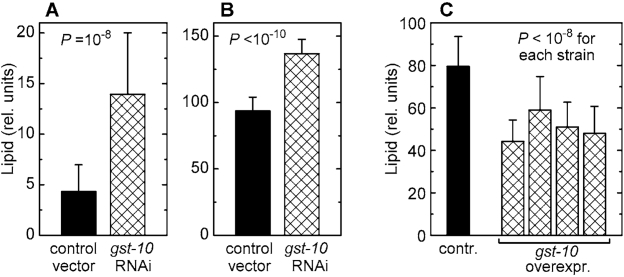
Figure 2. Lipid content of C. elegans with altered expression of gst‑10. (A) and (B): worms
fed bacteria carrying the insert-free L4440 feeding vector (control) and
worms subjected to RNAi against gst‑10. Lipid content was
measured by Nile red fluorescence (A) and by Sudan black staining (B).
(C): lipid content (by Sudan black) in worms
transformed with the insert-free pPD49.26 vector (control) and in four
transgenic C. elegans lines that overexpress gst‑10.
Bars represent means ± S.D. (n = 50 worms per group) of pixel intensities
integrated on worm micrographs.
Disruption of oxidative metabolism of 4‑hne leads to a fat
accumulation phenotype resembling that seen in 4‑hne
conjugation-defective
In addition to glutathione conjugation, 4‑HNE can undergo
reductive or oxidative metabolism (reviewed in refs. [5, 6]). In mammalian cells,
several aldehyde dehydrogenases that catalyze the oxidation of the carbonyl
group of 4‑HNE are physiologically important [2,26,27]. Of the twelve
aldehyde dehydrogenase (alh) genes of C. elegans (WormBase
release WS194), we investigated alh-1 which has a high, and alh-10 which has a moderate contribution to whole-body NAD+-dependent 4‑HNE
oxidation (Figure 3A). Silencing of the alh-1 gene led to a
statistically significant increase in fat accumulation, as measured either by
Nile red (Figure 3B) or by Sudan black (Figure 3C) staining. In contrast, RNAi
against alh-10 had no effect on the level of stored lipids (Figure 3B
and C). These results show that the inverse relationship between the
accumulation of fat and the organism's capacity to metabolize 4‑HNE is not limited to glutathione
conjugation of 4‑HNE by the gst‑10 gene product, but that it
extends to another type of 4‑HNE metabolism catalyzed by an unrelated
enzyme.
Direct exposure of C. elegans to synthetic 4‑HNE leads
to an increase of 4‑HNE-protein adducts and causes the fat accumulation
phenotype
Treatment of C. elegans in liquid culture with chemically synthesized 4‑HNE caused an increase in
the level of 4‑HNE-protein adducts (Figure 4). This increase was greater
than that seen in worms subjected to RNAi against gst‑10 (Figure 1),
possibly because gst-10 expression is restricted to relatively few cells
of C. elegans [22]. Given the limitations on long-range diffusion
of 4‑HNE [28], the effects on 4‑HNE that result from gst-10 disruption may also be local, and would appear as moderate when averaged over
the entire organism. In contrast, most or all tissues are likely to be affected
in worms exposed to 4‑HNE-containing
medium. In addition to the tissue distribution of 4‑HNE, the relatively high concentration (1 mM) of the compound
in the growth medium could have also contributed to the pronounced
increase in adducts. However, it is important to note that the biologically
active concentration of 4‑HNE in tissues of C. elegans is
approximately three orders of magnitude lower than the concentration of
externally added 4‑HNE [20]. Thus, the effective 4‑HNE
concentration was likely on the order of 1 μM,
well within a physiologically achievable range (low micromolar; 5).
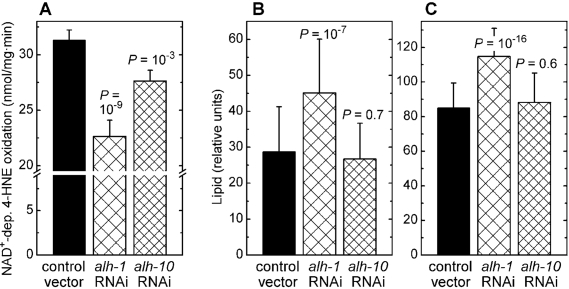
Figure 3. Effects of RNAi against aldehyde dehydro-genases alh-1 and alh-10. (A) Effect of RNAi against alh-1 and alh-10 on NAD+-dependent 4‑HNE
oxidation capacity of worm homogenates.
(B) and (C): Fat content
in C. elegans subjected to RNAi against alh-1 and alh-10,
determined by Nile red (A) and Sudan black (B)
staining followed by pixel integration. Means ± S.D. (n = 51 worms per
group) are shown. In all experiments, statistical significance versus
insert-free L4440 feeding vector control was assessed by the t‑test
with Bonferroni correction.
Exposure of C. elegans to synthetic 4‑HNE led to storage of excess lipids that increased with
increasing medium concentration of 4‑HNE, as shown by either Nile red or
Sudan black staining (Figure 5A and B, respectively). No significant
fluorescence was visible in the absence of Nile red (Figure 5A, upper row of
images), demonstrating that the observed signal is not caused by increased
autofluorescence in response to the treatment with 4‑HNE. This is
important because 4‑HNE has been implicated in the process of lipofuscin
formation [29]. Similarly, there was no signal in brightfield images of worms
treated with 4‑HNE but not stained (Figure 5B, upper row), whereas
identically treated animals that were stained with Sudan black yielded a strong
signal under the same exposure conditions (Figure 5B, lower row of images).
Quantitation of multiple images shows that the amount of accumulated fat was
linearly proportional to the medium concentration of 4‑HNE in the range
of 0.25 - 2 mM (Figure 5C).
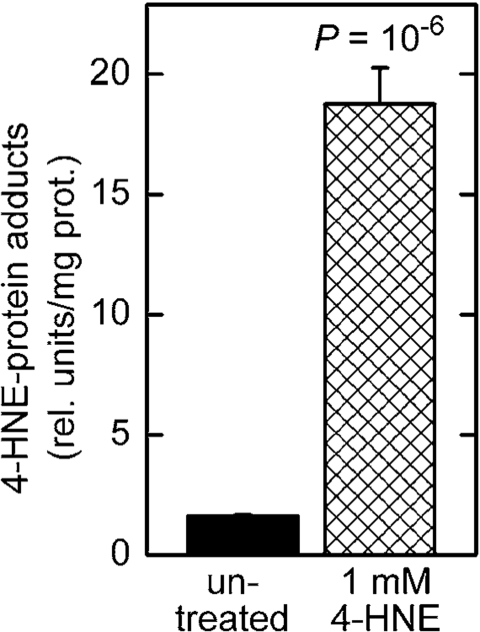
Figure 4. Increased level of 4‑HNE-protein adducts in C. elegans treated with 4‑HNE. Synchronized L4 worms were
kept in liquid culture for 3 days in medium containing 1 mM 4‑HNE.
The medium was replaced every 12 hr with fresh medium containing 4‑HNE.
The level of 4‑HNE-protein adducts was determined in 5-day-old worms
by competitive ELISA [93]. The values shown are means ± S.D. of 4
biological replicates (independently grown batches of worms) per group.
Fat accumulation triggered by 4‑HNE is
accompanied by metabolic changes consistent with increased de novo synthesis and/or decreased β-oxidation of
fatty acids
Malonyl-CoA is a metabolite of key importance in lipid
homeostasis (see Discussion). Malonyl-CoA was measured in C. elegans in which the level of 4‑HNE was increased by either of two methods:
direct treatment with 4‑HNE, or RNAi against gst‑10 which
leads to an impairment of 4‑HNE removal. In both situations, the
concentration of malonyl-CoA was elevated (Figure 6A and B). Worms treated in
the same way were also assayed for citrate, a precursor and activator of
malonyl-CoA synthesis (see Discussion). In analogy to malonyl-CoA, the
concentration of citrate was increased as a consequence of diminished metabolic
elimination of 4‑HNE or in response to direct supplementation of worms
with 4‑HNE (Figure 7).
Discussion
Experimental manipulation of enzymes that
catalyze elimination of 4‑HNE from tissues modulates the steady-state
level of 4‑HNE and affects the amount of stored fat in C. elegans.
Specifically, inhibition of either conjugation or oxidation of 4‑HNE
leads to increased fat accumulation, whereas overexpression of a 4‑HNE-conjugating
enzyme results in a lean phenotype (Figure 2 and Figure 3). These observations
are consistent with the obese phenotype we have previously reported for mGsta4 null mice which have an impaired ability to conjugate 4‑HNE [19],
indicating that the link between abundance of 4‑HNE and fat storage is
conserved between evolutionarily distant species.
We propose that 4‑HNE, rather than another substrate
or function of the enzymes that are being experimentally manipulated, is a
causative factor in the enhanced storage of lipid. We favor this model for the
following reasons:(i) Except for 4‑HNE,
there is no known common substrate of the two rather dissimilar C. elegans enzymes used in the present work, i.e., the products of gst‑10 and alh‑1 genes. Moreover, 4‑HNE is the only known common
substrate of the above-mentioned two enzymes and the murine mGSTA4-4 [30].
(ii) Fat
deposition was reported for yeast directly treated with 4‑HNE [31], an
experimental design that points to 4‑HNE as the causative agent.
(iii) mGsta4 null mice in the 129/sv
genetic background have an elevated tissue levels of 4‑HNE and are obese, while mGsta4 null mice in C57BL
genetic background have near-normal levels of 4‑HNE and are not obese [19]. The proportionality of 4‑HNE and fat deposition in
otherwise closely related systems suggests a causal role of 4‑HNE in lipid accumulation in the
murine model.
(iv) Overexpression of murine aldose reductase
Akr1b7 [an enzyme catalyzing reductive metabolism of 4-HNE, ref. 32] inhibits
adipogenesis, and disruption of the same enzyme accelerates adipogenesis [33].
Concordant results obtained with mGSTA4-4 and Akr1b7 indicate that a common
substrate, such as 4‑HNE, is
responsible for the phenotype.
While alternative
explanations could be invoked for each of the findings listed above, a strong
case for a causal involvement of 4‑HNE
can be made if the arguments are considered in aggregate. It is unlikely that
several enzymes that act on distinct functional groups of 4‑HNE would all
share a hypothetical additional substrate. The reactions these enzymes catalyze
include Michael addition of a nucleophile to a polarized double bond (GSTs),
NAD+-dependent oxidation of an aldehyde to a carboxylic acid
(aldehyde dehydrogenase), and NAD(P)H-dependent reduction of
an aldehyde to an alcohol (aldose
reductase). To be able to undergo all three reactions, a substrate must contain
the requisite target functional groups; this requirement restricts the set of
possible substrates to α,β-unsaturated carbonyl compounds
such as 4‑HNE. Moreover,
experimental interventions that lower the expression of 4‑HNE-conjugating GSTs in C. elegans (gst-10)
and in mice (mGSTA4-4) resulted in a similar phenotype, i.e., increased
fat storage. The two enzymes are not orthologs, and their ability to conjugate 4‑HNE probably evolved independently
[22,34]. It is unlikely that both enzymes acquired by chance another shared activity
or function that is unrelated to conjugation of 4‑HNE, indicating that the parallel effects of disruptingmGSTA4-4 and gst-10 are indeed mediated by 4‑HNE.
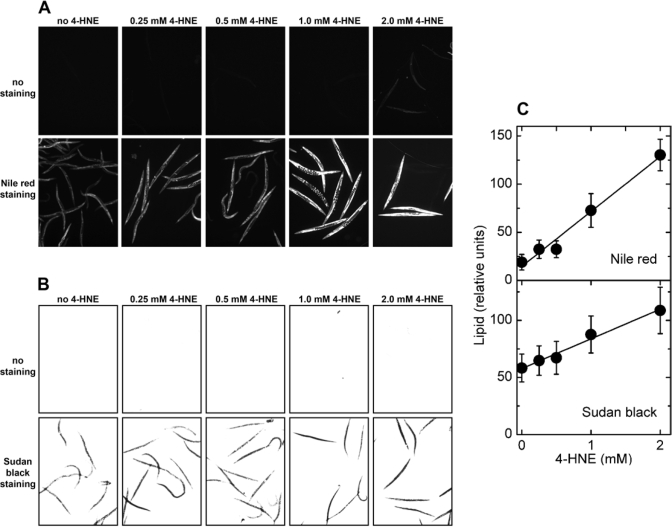
Figure 5. Effects of direct treatment with 4‑HNE on lipid accumulation in C. elegans.
Synchronized L4 worms were kept in liquid
culture for 3 days in medium containing the indicated concentration of 4‑HNE.
The medium was replaced every 12 hr with fresh medium containing 4‑HNE.
(A) fluorescence of worms treated with the indicated
concentration of 4‑HNE but not stained (upper row of images),
and animals exposed to 4‑HNE and stained with Nile red (lower row
of images). (B) brightfield images of worms treated with
the indicated concentration of 4‑HNE and directly photographed (upper
row of images), or photographed after staining with Sudan black (lower row of images).
(C): for each 4‑HNE concentration, lipid
content was measured in three independently treated worm populations by
quantitation of Nile red fluorescence (upper plot) or Sudan black
staining (lower plot).
As outlined above,
experimental modulation of 4‑HNE-metabolizing
enzymes indicates that 4‑HNE
can trigger fat accumulation. However, even though highly persuasive, the
evidence is indirect. The existence of an alternative substrate of the three 4‑HNE-metabolizing enzymes is
unlikely but cannot be ruled out with certainty. In addition, decreasing the
expression of the three enzymes could lead to lipid accumulation via three
independent mechanisms, none of them involving 4‑HNE - a hypothesis that is implausible but not impossible.
Therefore, we wished to test a possible causative role of 4‑HNE without relying on enzymes.
For this purpose, we took advantage of the unique experimental opportunities
afforded by C. elegans, a multicellular animal that can be handled
by methods developed for microorganisms or cultured cells [35]. This includes
the option to grow C. elegans in liquid medium that can be
supplemented with compounds to be tested. Exposure of C. elegans to synthetic 4‑HNE
led to dose-dependent lipid deposition (Figure 5) similar to that triggered by
silencing of either gst-10 or alh-1. While this finding does not
formally rule out the possibility that hypothetical alternative substrates of gst-10 and alh-1 are also involved, it proves that 4‑HNE is sufficient to elicit the fat accumulation
phenotype.
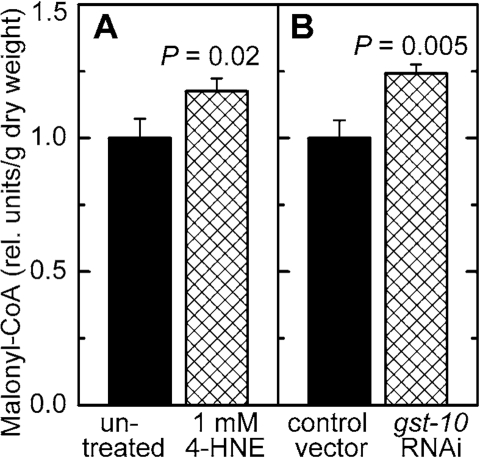
Figure 6. Effect of increased 4‑HNE levels on malonyl-CoA concentration. C. elegans were either treated with 1 mM 4‑HNE according to the protocol
described in the legend of Figure 5(A) or subjected to RNAi
to gst‑10(B)). The results were normalized to the
respective control and are shown as mean ± S.D. of 3 independently grown
worm populations. In (A), the control (C. elegans Bristol-N2 in liquid culture in the presence of E. coli OP50 but
without 4‑HNE) contained 16.5 ± 1.2 ng malonyl-CoA/mg dry weight;
in (B), the level of malonyl-CoA in the control (C. elegans Bristol-N2 grown on plates seeded with E. coli HT115(DE3)
transformed with insert-free L4440 feeding vector) was 12.2 ± 0.8 ng/mg dry
weight.
The increase in stored fat was similar
for worms directly exposed to 4‑HNE (Figure 5) and for those with
silenced 4‑HNE-metabolizing enzymes (Figure 2 and Figure 3), even though
the former treatment was markedly more effective in raising the level of 4‑HNE-protein
adducts (Figure 4 versus Figure 1). These findings could indicate that
silencing gst‑10 or alh-1 raises the concentration of 4‑HNE
in selected tissues, including those involved in regulating fat storage. In
contrast, exposure of animals to external 4‑HNE is likely to affect the
entire body. Either treatment would reach the critical target tissues and
trigger fat accretion, but exposure to 4‑HNE in the medium would generate
more total 4‑HNE adducts. Further work is needed to identify the cells or
tissues that are requires for increased fat storage in response to 4‑HNE,
although head neurons are a possibility. Head neurons express the gst‑10 [22] as well as the alh-1 gene products (WormBase release WS194).
What is the molecular mechanism by which 4‑HNE
increases the level of stored fat? A clue is offered by our finding that the
concentration of malonyl-CoA was increased in C. elegans with
disrupted enzymatic removal of 4‑HNE, as well as in animals directly
exposed to 4‑HNE (Figure 6). Malonyl-CoA has a dual metabolic function:
it is the substrate for fatty acid synthesis, and it prevents fatty acid β-oxidation [reviewed in ref. 36]. Therefore, elevated malonyl-CoA
levels lead to an accumulation of fatty acids and, consequently, of
triglycerides. In fact, experimental
lowering of malonyl-CoA levels results in a lean phenotype in mammals [37-39]
as well as in C. elegans [40]. Conversely, liver-specific disruption of
AMPK, expected to increase malonyl-CoA levels, results in elevated hepatic
lipogenesis [41,42]. These data indicate that the higher level of malonyl-CoA
that we observed in worms with increased 4‑HNE could account for the
concomitant fat accumulation.
Malonyl-CoA is formed from acetyl-CoA by the enzyme
acetyl-CoA carboxylase (ACC). Although the steady-state tissue concentration of
malonyl-CoA depends on the rate of its synthesis by ACC as well as its removal
rate by fatty acid synthase or by malonyl-CoA decarboxylase, the activity of
ACC is considered to be the major physiological determinant of malonyl-CoA
levels at least in some tissues [43]. Therefore, the regulation of ACC activity
is of central importance for fat homeostasis. Not surprisingly, ACC has been
proposed as a target for pharmacological intervention [39,44].
Regulation of ACC is mostly
post-translational. In mammals, a major regulatory mode is phosphorylation,
particularly by AMPK [45,46]. Although C.
elegans ACC lacks the canonical target sequence for AMPK phosphorylation [47],
worm ACC is probably still regulated by AMPK, perhaps using an alternative
phosphorylation site, because AMPK is required for metabolic modulation of fat
stores [48]. Further work will be needed to elucidate the details of ACC
phosphorylation in C. elegans.
In addition to phosphorylation, ACC is acutely
regulated by allosteric effectors including citrate (reviewed in refs. [47, 49]).
In the well-fed state, excess citrate is withdrawn from the TCA cycle and
transported out of mitochondria. In the cytosol, citrate has a dual function in
fat synthesis: (i) it allosterically activates ACC, and (ii) it is converted by
citrate lyase to acetyl-CoA, the substrate of ACC (Figure 8). The resulting
higher flux through ACC increases the supply of malonyl-CoA.
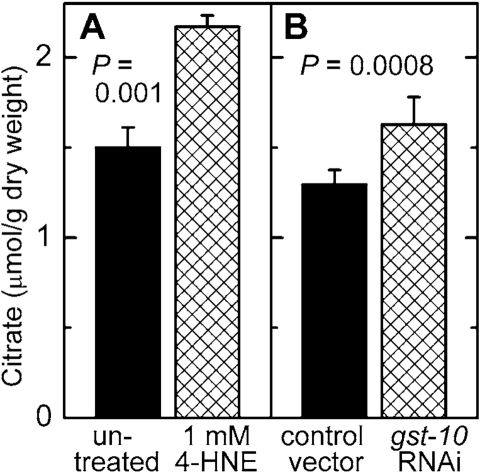
Figure 7. Effect of increased 4 HNE levels on citrate concentration. C. elegans were either treated with 1 mM 4- HNE
according to the protocol described in the legend of Figure 5(A)
or subjected to RNAi to gst 10 (B). The results are shown as
means ± S.D. of 3 to 6 independently grown worm populations.
A partial
inhibition of the tricarboxylic acid cycle enzyme aconitase lead to increased
steady-state levels of citrate [50]. This is relevant to the present work
because aconitase is susceptible to inhibition by 4‑HNE. It has been
reported that adducts of 4‑HNE with aconitase form when isolated
mitochondria are exposed to 4‑HNE
[ref. 51 and references therein], and that treatment of cultured cells with 4‑HNE leads to a decrease in
aconitase activity [52]. We found that mGsta4 knockout mice have lower
aconitase activity and higher citrate levels than wild-type animals [19]. We
now demonstrate that increasing the levels of 4‑HNE, either by direct
treatment with the compound or by disruption of the metabolic disposal of 4‑HNE,
results in an elevated citrate concentration in C. elegans (Figure 7).
Thus, our results support a model in which 4‑HNE partially inhibits
aconitase, causing an increase in the steady-state concentration of citrate.
The latter metabolite augments the flux through ACC by providing both the
substrate and the allosteric activator of the enzyme. This leads to more
malonyl-CoA production, and thus to more fatty acid synthesis and less fatty
acid β-oxidation. The
accumulating fatty acids are converted to storage fat. The model is
schematically depicted in Figure 8.
We propose that the
chain of events leading from 4‑HNE
to increased fat storage operates not only when 4‑HNE levels are
experimentally perturbed, but that it is part of normal physiology. In the
wild, access to food is typically intermittent. It is therefore highly adaptive
to maximize stores of metabolic fuel when food is available. Such stores
increase the probability of surviving "lean" periods. Feeding triggers the
synthesis and deposition of fat. At least in mammals, increased fat storage
correlates with a more pronounced pro-inflammatory and pro-oxidative state
[53, 54]. Oxidative stress is expected to facilitate lipid peroxidation and raise 4‑HNE levels. In fact, it has been
shown directly that feeding-induced obesity in mice causes an increase in
tissue concentration of 4‑HNE
[14]. In turn, elevated 4‑HNE
realigns metabolism to favor fat deposition by increasing malonyl-CoA
production, as described by us in the present report. We propose that these two
partial processes - fat-induced 4‑HNE
generation and 4‑HNE-triggered
fat accumulation - form a positive feedback loop that perpetuates lipid storage
as long as food is available. The proposed self-reinforcing loop is
schematically depicted in Figure 8.
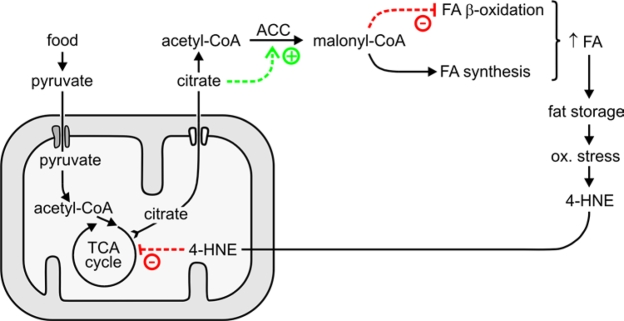
Figure 8. Proposed role of 4‑HNE in the generation and maintenance of fat stores.
Although self-sustaining
while food is available, the postulated regulatory circuit involving 4‑HNE
is not irreversible. When food becomes scarce (i.e., under caloric
restriction conditions), metabolic and sensory inputs would act to shift
metabolism to a mode that emphasizes somatic maintenance and utilization rather
than storage of fat [55-57]. In this physiologic state, ROS generation is
decreased, perhaps because of altered mitochondrial function [58]. Less ROS
translates into less 4‑HNE production. At the same time, decreased
insulin-like signaling would augment the Daf-16-dependent expression of the gst‑10 gene that encodes a 4‑HNE-conjugating enzyme [21,22]. The combination of
limited supply and enhanced removal would lower the concentration of 4‑HNE,
thus decreasing the production of malonyl-CoA by reversing the mechanisms
summarized in Figure 8. This would, in turn, limit fatty acid synthesis and
permit fatty acid β-oxidation, as required
for the utilization of fat as a metabolic fuel.
The mechanism proposed
in Figure 8 suggests that the metabolic "pro-adipose bias" observed in the
well-fed state can be reversed not only by withdrawal of food, but also by
withdrawal of 4‑HNE, even in the continuing presence of excess food.
Pharmacological agents that act as 4‑HNE scavengers have been reported [59-64].
Clearly, the 4‑HNE-mediated mechanism we propose is not the only process
that favors fat accumulation. Nevertheless, the potential importance of
modulating 4‑HNE levels in preventing or treating obesity could be
considerable.
Modulation of gst‑10 expression has opposite effects on fat accumulation and on life span of C. elegans. Worms with silenced gst‑10 (and
elevated 4‑HNE) accumulate fat (Figure 2) and have a shortened life span
[21], whereas overexpression of gst‑10 results in a lean phenotype
(Figure 2) and it prolongs life [22]. In mammals, on an epidemiological level
obesity correlates with an increased risk for metabolic syndrome and earlier
mortality [reviewed in ref. 65]; it has been recently suggested that oxidative
stress-triggered fat deposition not only correlates with, but causes aging [66].
However, the link between fat accumulation, disease, and longevity, while
undeniable, is complex and defies simple generalizations. The tissue
distribution of lipid is an important factor. Recent data indicate that the primary trigger of metabolic syndrome in
mammals is not total but ectopic fat, and that fat in adipose tissue may play a
lesser role or could actually be protective by preventing the accretion of
ectopic fat [65,67-71]. Another factor that is relevant to longevity is
the chemical nature of lipids, in particular the degree of unsaturation of fatty
acids and the position of double bonds; highly peroxidizable polyunsaturated
fatty acids may limit life span [72-77]. In C. elegans, fat
accumulation has been found to be associated with either shortened or extended
life span [48,78-82], probably reflecting
differences in fat localization, structure, or timing of fat abundance in the
course of development. Recent work has demonstrated that lipid stores provide a
metabolic link in the signaling pathway from germline stem cells to longevity,
as well as in the mechanism by which lower Daf-2 activity leads to an extension
of life [83]. In the latter work, the decreased fat stores that were associated
with a longer life span were caused by induction of a triglyceride lipase. We
report the same inverse relationship between fat and longevity when lipid
stores are modulated by altering the supply of fatty acids [this work and refs.
20-22]. Therefore, our data support the notion that accumulation of fat,
whether through increased synthesis or lowered utilization, can shorten life
span of C. elegans.
In summary, we have
identified a biochemical mechanism, mediated through the lipid peroxidation
product 4‑HNE, that promotes fat accumulation. The mechanism is part of a
positive feedback loop that confers a pro-adipose bias on organismal lipid
homeostasis. The proposed mechanism differs from the better-known endocrine
regulation of fat-related physiology by its purely biochemical nature.
Therefore, the mechanism may offer heretofore unexplored opportunities for
intervention.
Materials and Methods
C. elegans
culture conditions.
C. elegans Bristol-N2 were cultured at 20°C on 2% agar plates containing nematode growth
medium (NG medium: 25 mM potassium phosphate, pH 6.0, 50 mM NaCl, 0.6% (w/v)
peptone, 5 μg/ml of cholesterol, 1 mM MgSO4,
1 mM CaCl2) and seeded with Escherichia coli strain OP50 [modified from ref. 84]. E. coli OP50 were
grown in LB medium (10 g/l of Bacto-Tryptone, 5 g/l of Yeast Extract, 10 g/l of
NaCl, pH 7.0).
The generation and maintenance of transgenic C. elegans overexpressing gst‑10 was described previously [22]. In experiments employing RNAi, C. elegans Bristol-N2 worms were fed E. coli strain HT115(DE3) transformed with an insert-free L4440 feeding vector
(control), or the same vector carrying the appropriate insert. Specifically,
RNAi against gst‑10 was conducted according to [21]. RNAi against alh-1 and alh-10 was carried out using clones III-2N01 and X-3B18,
respectively, from the RNAi library [85,86] purchased from Geneservice Ltd, Cambridge, U.K. To induce the expression of double-stranded RNA, bacteria were treated
with 1 mM isopropyl-β-d-thiogalacto-pyranoside
(IPTG).
For liquid culture, synchronized C. elegans at the L4 stage (2.5 days post-hatch) were rinsed off plates and approximately
1,500 worms were placed in 3 ml S-buffer [0.1 M NaCl, 0.05 M potassium
phosphate, pH 6.0; ref. 84] supplemented with cholesterol (3 μl of a 5 mg/ml stock solution in ethanol) and with the same E. coli strain that was used for the initial growth on plates at a final density of A600= 1.0. The cultures were maintained at 20°C except for gst-10 overexpressors
which were grown at 25°C. The medium was replaced every 12 hours with fresh
medium containing the components listed above, including fresh bacteria.
Treatment of C. elegans with 4‑HNE.
Worms were
maintained in liquid culture for 3 days under the conditions described above,
except that 4‑HNE (at concentrations ranging from zero to 2.0 mM) was
added to the growth medium. Therefore, worms were exposed to fresh 4‑HNE
every 12 hours for a total of three days.
Nile red and Sudan black staining of C. elegans. For Nile red staining, worms were
grown in liquid culture in medium supplemented with 75 ng/ml Nile Red (added as
a 0.5 mg/ml stock solution in acetone). After 3 days of treatment with Nile red
and, where used, with concurrently added 4‑HNE, worms were washed for 3
× 10 min with S-buffer, and were placed on a slide and covered with a
coverslip. Randomly selected fields were photographed under a fluorescence
microscope (Nikon Eclipse E1000 equipped with a digital camera) using a
4× objective and an identical exposure time (300 ms) for all images. The
filter cube consisted of a 465-495 nm excitation filter, a 505 nm long pass
dichroic mirror, and a 540 nm long pass emission filter. Fluorescence spectra
of Nile red-stained lipids of different polarity [23,24,87] indicate that
this filter combination registers both neutral and polar lipids, although
excitation for polar lipids is suboptimal. Therefore, under the conditions
used, mostly neutral fat is quantitated.
For Sudan black B staining [88], worms were
synchronized and processed as described in the preceding paragraph, except that
Nile red was omitted from the solutions. After three days in liquid culture,
worms were washed in S-buffer for 30 min, fixed with 1% paraformaldehyde in S
buffer, and subjected to three freeze-thaw cycles. The animals were then
dehydrated through consecutive washes with 25%, 50%, and 70% ethanol. Staining
was performed overnight (approximately 16 hours) in a 50% saturated solution of
Sudan black B in 70% ethanol. Following staining, worms were washed for 4
× 10 min with M9 buffer [6 g Na2HPO4, 3 g KH2PO4,
5 g NaCl and 0.25 g MgSO4·7H2O per liter; ref. 84] and
randomly chosen fields were photographed under a brightfield microscope (Nikon
Eclipse E1000 equipped with a digital camera) with Nomarski optics and a
4× objective. The exposure time (10 ms) was identical for all images.
All images were saved as monochrome 16-bit TIFF files,
and were analyzed in the ImageJ program http://rsbweb.nih.gov/ij. Files were
converted to 8-bit depth, were inverted for Sudan black stains, and the
perimeter of 50 to 100 non-overlapping, complete worm images was traced
manually. The mean pixel intensity was recorded for each scored animal after
background subtraction. The mean intensity represents the amount of dye, and
thus amount of lipid, per unit of surface area in the image, i.e., the
density of lipid in the organism. This lipid density parameter corresponds to
that used by Ashrafi et al. [40], except that it is averaged over the entire
organism rather than over a chosen region of the worm body. We also calculated
a related parameter, the total amount of fat in an organism, by multiplying the
mean pixel intensity by the number of pixels in the image of each worm.
Analysis of the total lipid content (not shown) and of the lipid density
presented in the results section yielded the same conclusions.
Enzyme assays
. Conjugation of 4‑HNE
with gluta-thione (GST activity) was measured according to Alin et al. [89] in
whole-body homogenates of C. elegans obtained as described
previously [20]. For the determination of NAD+-dependent oxidation
of 4‑HNE (aldehyde dehydrogenase activity), worms from four 100-mm plates
(approximately 10,000 animals) were washed with 50 mM Tris-HCl, pH 7.4, 5 mM
EDTA, and stored at -70°C. Immediately before use, 0.1 ml of the above buffer
were added to the worm pellet, and the suspension was sonicated on ice for 3
× 10 s and centrifuged for 20 min at 12,000 g at 4°C. Aldehyde
dehydrogenase activity was assayed in the supernatant according to [90], except
that 1 mM 4‑HNE was used as the substrate instead of benzaldehyde, and 1
mM NAD+ was used as the co-substrate instead of NADP+.
Determination of metabolite levels.
For the
determination of citrate, frozen worms were homogenized in 1 M perchloric
acid. Following centrifugation, the supernatant was neutralized and citrate
was measured by a coupled enzyme assay utilizing citrate lyase and malate and
lactate dehydrogenases [91], as implemented in the citric acid determination
kit by Boehringer Mannheim/ R-Biopharm (Roche, Marshall, MI).
To assay malonyl-CoA, worm pellets were frozen and
stored at -70°C. On the day of assay, the pellets were pulverized in a mortar
at liquid nitrogen temperature, and were lyophilized overnight. The weight of
the powder was recorded, and the samples were processed and malonyl-CoA was
determined by HPLC by a modification of the method of Lazzarino et al.[92] as described by us previously [19].
Assay of 4‑HNE-protein
adducts.
The level of 4‑HNE-modified
proteins was determined by competitive ELISA [93] as modified by us previously
[20].
Materials.
4‑HNE dimethylacetal was
synthesized according to [94,95]. 4‑HNE
was prepared on the day of use by hydrolysis of 4‑HNE dimethylacetal in 1 mM HCl for 1 hour at room
temperature. Sudan black B was from Aldrich (Milwaukee, WI; catalog number
19,966-4), and Nile red was from Molecular Probes (Carlsbad, CA; catalog number
N-1142).
Acknowledgments
This
work was supported in part by National Institutes of Health grants R01 AG028088
and AG018845 (to P.Z.). P.Z. is a recipient of a VA Research Career Scientist
Award.
Conflicts of Interest
The
authors declare no conflict of interest.
References
-
1.
Guichardant
M
, Bacot
S
, Moliere
P
and Lagarde
M.
Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites.
Prostaglandins Leukot Essent Fatty Acids.
2006;
75:
179
-182.
[PubMed]
.
-
2.
Long
EK
, Murphy
TC
, Leiphon
LJ
, Watt
J
, Morrow
JD
, Milne
GL
, Howard
JR
and Picklo
MJ Sr.
Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation.
J Neurochem.
2008;
105:
714
-724.
[PubMed]
.
-
3.
Esterbauer
H
, Schaur
RJ
and Zollner
H.
Chemistry and bio-chemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes.
Free Radic Biol Med.
1991;
11:
81
-128.
[PubMed]
.
-
4.
Grimsrud
PA
, Xie
H
, Griffin
TJ
and Bernlohr
DA.
Oxidative stress and covalent modification of protein with bioactive aldehydes.
J Biol Chem.
2008;
283:
21837
-21841.
[PubMed]
.
-
5.
Poli
G
, Schaur
RJ
, Siems
WG
and Leonarduzzi
G.
4-Hydro-xynonenal: a membrane lipid oxidation product of medicinal interest.
Med Res Rev.
2008;
28:
569
-631.
[PubMed]
.
-
6.
Petersen
DR
and Doorn
JA.
Reactions of 4-hydroxynonenal with proteins and cellular targets.
Free Radic Biol Med.
2004;
37:
937
-945.
[PubMed]
.
-
7.
Leonarduzzi
G
, Robbesyn
F
and Poli
G.
Signaling kinases modulated by 4-hydroxynonenal.
Free Radic Biol Med.
2004;
37:
1694
-1702.
[PubMed]
.
-
8.
Dwivedi
S
, Sharma
A
, Patrick
B
, Sharma
R
and Awasthi
YC.
Role of 4-hydroxynonenal and its metabolites in signaling.
Redox Rep.
2007;
12:
4
-10.
[PubMed]
.
-
9.
Forman
HJ
, Fukuto
JM
, Miller
T
, Zhang
H
, Rinna
A
and Levy
S.
The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal.
Arch Biochem Biophys.
2008;
477:
183
-195.
[PubMed]
.
-
10.
Rajala
MW
and Scherer
PE.
The adipocyte - at the crossroads of energy homeostasis, inflammation, and atherosclerosis.
Endocrinology.
2003;
144:
3765
-3773.
[PubMed]
.
-
11.
Cinti
S
, Mitchell
G
, Barbatelli
G
, Murano
I
, Ceresi
E
, Faloia
E
, Wang
SL
, Greenberg
AS
and Obin
MS.
Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans.
J Lipid Res.
2005;
46:
2347
-2355.
[PubMed]
.
-
12.
Berg
AH
and Scherer
PE.
Adipose tissue, inflammation, and cardiovascular disease.
Circulation Res.
2005;
96:
939
-949.
[PubMed]
.
-
13.
Russell
AP
, Gastaldi
G
, Bobbioni-Harsch
E
, Arboit
P
, Gobelet
C
, Deriaz
O
, Golay
A
, Witztum
JL
and Giacobino
J-P.
Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids.
FEBS Lett.
2003;
551:
104
-106.
[PubMed]
.
-
14.
Grimsrud
PA
, Picklo
Sr MJ
, Griffin
TJ
and Bernlohr
DA.
Carbonylation of adipose proteins in obesity and insulin resistance: Identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal.
Mol Cell Proteomics.
2007;
6:
624
-637.
[PubMed]
.
-
15.
Awasthi
YC
, Sharma
R
, Sharma
A
, Yadav
S
, Singhal
SS
, Chaudary
P
and Awasthi
S.
Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death.
Free Radic Biol Med.
2008;
45:
111
-118.
[PubMed]
.
-
16.
Kutuk
O
and Basaga
H.
Apoptosis signalling by 4-hydro-xynonenal: a role for JNK-c-Jun/AP-1 pathway.
Redox Rep.
2007;
12:
30
-34.
[PubMed]
.
-
17.
Echtay
KS
and Brand
MD.
4-hydroxy-2-nonenal and uncoupling proteins: an approach for regulation of mitochondrial ROS production.
Redox Rep.
2007;
12:
26
-29.
[PubMed]
.
-
18.
Barrera
G
, Pizzimenti
S
and Dianzani
MU.
Lipid peroxidation: control of cell proliferation, cell differentiation and cell death.
Mol Aspects Med.
2008;
29:
1
-8.
[PubMed]
.
-
19.
Singh
SP
, Niemczyk
M
, Saini
D
, Awasthi
YC
, Zimniak
L
and Zimniak
P.
Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice.
Biochemistry.
2008;
47:
3900
-3911.
[PubMed]
.
-
20.
Ayyadevara
S
, Dandapat
A
, Singh
SP
, Siegel
ER
, Shmookler
Reis RJ
, Zimniak
L
and Zimniak
P.
Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal.
Mech Ageing Dev.
2007;
128:
196
-205.
[PubMed]
.
-
21.
Ayyadevara
S
, Dandapat
A
, Singh
SP
, Beneš
H
, Zimniak
L
, Shmookler
Reis RJ
and Zimniak
P.
Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2.
Aging Cell.
2005;
4:
299
-307.
[PubMed]
.
-
22.
Ayyadevara
S
, Engle
MR
, Singh
SP
, Dandapat
A
, Lichti
CF
, Beneš
H
, Shmookler
Reis RJ
, Liebau
E
and Zimniak
P.
Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal.
Aging Cell.
2005;
4:
257
-271.
[PubMed]
.
-
23.
Greenspan
P
and Fowler
SD.
Spectrofluorometric studies of the lipid probe, nile red.
J Lipid Res.
1985;
26:
781
-789.
[PubMed]
.
-
24.
Greenspan
P
, Mayer
EP
and Fowler
SD.
Nile red: a selective fluorescent stain for intracellular lipid droplets.
J Cell Biol.
1985;
100:
965
-973.
[PubMed]
.
-
25.
McKay
RM
, McKay
JP
, Avery
L
and Graff
JM.
C. elegans: a model for exploring the genetics of fat storage.
Dev Cell.
2003;
4:
131
-142.
[PubMed]
.
-
26.
Marchitti
SA
, Orlicky
DJ
and Vasiliou
V.
Expression and initial characterization of human ALDH3B1.
Biochem Biophys Res Commun.
2007;
356:
792
-798.
[PubMed]
.
-
27.
Townsend
AJ
, Leone-Kabler
S
, Haynes
RL
, Wu
Y
, Szweda
L
and Bunting
KD.
Selective protection by stably transfected human ALDH3A1 (but not human ALDH1A1) against toxicity of aliphatic aldehydes in V79 cells.
Chem Biol Interact.
2001;
130-132:
261
-273.
[PubMed]
.
-
28.
Singh
SP
, Chen
T
, Chen
L
, Mei
N
, McLain
E
, Samokyszyn
V
, Thaden
JJ
, Moore
MM
and Zimniak
P.
Mutagenic effects of 4-hydroxynonenal triacetate, a chemically protected form of the lipid peroxidation product 4-hydroxynonenal, as assayed in L5178Y/Tk+/- mouse lymphoma cells.
J Pharmacol Exp Ther.
2005;
313:
855
-861.
[PubMed]
.
-
29.
Schutt
F
, Bergmann
M
, Holz
FG
and Kopitz
J.
Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium.
Invest Ophthalmol Vis Sci.
2003;
44:
3663
-3668.
[PubMed]
.
-
30.
Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, Hayden JB, Awasthi YC. Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4-4 (GST 5.7).
J Biol Chem.
1994;
269:
992
-1000.
[PubMed]
.
-
31.
Wonisch
W
, Zellnig
G
, Kohlwein
SD
, Schaur
RJ
, Bilinski
T
, Tatzber
F
and Esterbauer
H.
Ultrastructural analysis of HNE-treated Saccharomyces cerevisiae cells reveals fragmentation of the vacuole and an accumulation of lipids in the cytosol.
Cell Biochem Funct.
2001;
19:
59
-64.
[PubMed]
.
-
32.
Lefrancois-Martinez
A-M
, Tournaire
C
, Martinez
A
, Berger
M
, Daoudal
S
, Tritsch
D
, Veyssiere
G
and Jean
C.
Product of side-chain cleavage of cholesterol, isocaproaldehyde, is an endogenous specific substrate of mouse vas deferens protein, an aldose reductase-like protein in adrenocortical cells.
J Biol Chem.
1999;
274:
32875
-32880.
[PubMed]
.
-
33.
Tirard
J
, Gout
J
, Lefrancois-Martinez
AM
, Martinez
A
, Begeot
M
and Naville
D.
A novel inhibitory protein in adipose tissue: the aldo-keto-reductase AKR1B7. Its role in adipogenesis.
Endocrinology.
2007;
148:
1996
-2005.
[PubMed]
.
-
34.
Zimniak
P
and Singh
SP.
Awasthi YC.
Families of glutathione transferases
Boca Raton, FL
CRC Press
11
-26.
Toxicology of glutathione transferases.
2006;
.
-
35.
Brenner
S
The genetics of behaviour.
Br Med Bull.
1973;
29:
269
-271.
[PubMed]
.
-
36.
Saggerson
D
Malonyl-CoA, a key signaling molecule in mammalian cells.
Annu Rev Nutr.
2008;
28:
253
-272.
[PubMed]
.
-
37.
Abu-Elheiga
L
, Matzuk
MM
, Abo-Hashema
KA
and Wakil
SJ.
Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2.
Science.
2001;
291:
2613
-2616.
[PubMed]
.
-
38.
Boucher
A
, Lu
D
, Burgess
SC
, Telemaque-Potts
S
, Jensen
MV
, Mulder
H
, Wang
MY
, Unger
RH
, Sherry
AD
and Newgard
CB.
Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue.
J Biol Chem.
2004;
279:
27263
-27271.
[PubMed]
.
-
39.
Harwood
HJ Jr
Treating the metabolic syndrome: acetyl-CoA carboxylase inhibition.
Expert Opin Ther Targets.
2005;
9:
267
-281.
[PubMed]
.
-
40.
Ashrafi
K
, Chang
FY
, Watts
JL
, Fraser
AG
, Kamath
RS
, Ahringer
J
and Ruvkun
G.
Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes.
Nature.
2003;
421:
268
-272.
[PubMed]
.
-
41.
Andreelli
F
, Foretz
M
, Knauf
C
, Cani
PD
, Perrin
C
, Iglesias
MA
, Pillot
B
, Bado
A
, Tronche
F
, Mithieux
G
, Vaulont
S
, Burcelin
R
and Viollet
B.
Liver adenosine monophosphate-activated kinase-α2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin.
Endocrinology.
2006;
147:
2432
-2441.
[PubMed]
.
-
42.
Viollet
B
, Mounier
R
, Leclerc
J
, Yazigi
A
, Foretz
M
and Andreelli
F.
Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders.
Diabetes Metabol.
2007;
33:
395
-402.
.
-
43.
Reszko
AE
, Kasumov
T
, David
F
, Thomas
KR
, Jobbins
KA
, Cheng
JF
, Lopaschuk
GD
, Dyck
JR
, Diaz
M
, Des
Rosiers C
, Stanley
WC
and Brunengraber
H.
Regulation of malonyl-CoA concentration and turnover in the normal heart.
J Biol Chem.
2004;
279:
34298
-34301.
[PubMed]
.
-
44.
Curtis
R
, Geesaman
BJ
and DiStefano
PS.
Ageing and metabolism: drug discovery opportunities.
Nat Rev Drug Discov.
2005;
4:
569
-580.
[PubMed]
.
-
45.
Minokoshi
Y
, Kim
YB
, Peroni
OD
, Fryer
LG
, Muller
C
, Carling
D
and Kahn
BB.
Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase.
Nature.
2002;
415:
339
-343.
[PubMed]
.
-
46.
Takekoshi
K
, Fukuhara
M
, Quin
Z
, Nissato
S
, Isobe
K
, Kawakami
Y
and Ohmori
H.
Long-term exercise stimulates adenosine monophosphate-activated protein kinase activity and subunit expression in rat visceral adipose tissue and liver.
Metabolism.
2006;
55:
1122
-1128.
[PubMed]
.
-
47.
Barber
MC
, Price
NT
and Travers
MT.
Structure and regulation of acetyl-CoA carboxylase genes of metazoa.
Biochim Biophys Acta.
2005;
1733:
1
-28.
[PubMed]
.
-
48.
Schulz
TJ
, Zarse
K
, Voigt
A
, Urban
N
, Birringer
M
and Ristow
M.
Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress.
Cell Metabol.
2007;
6:
280
-293.
.
-
49.
Munday
MR
Regulation of mammalian acetyl-CoA carboxylase.
Biochem Soc Trans.
2002;
30:
1059
-1064.
[PubMed]
.
-
50.
Hassel
B
, Sonnewald
U
, Unsgard
G
and Fonnum
F.
NMR spectroscopy of cultured astrocytes: effects of glutamine and the gliotoxin fluorocitrate.
J Neurochem.
1994;
62:
2187
-2194.
[PubMed]
.
-
51.
Stevens
SM Jr
, Rauniyar
N
and Prokai
L.
Rapid characterization of covalent modifications to rat brain mitochondrial proteins after ex vivo exposure to 4-hydroxy-2-nonenal by liquid chromatography-tandem mass spectrometry using data-dependent and neutral loss-driven MS3 acquisition.
J Mass Spectrom.
2007;
42:
1599
-1605.
[PubMed]
.
-
52.
Raza
H
, John
A
, Brown
EM
, Benedict
S
and Kambal
A.
Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells.
Toxicol Appl Pharmacol.
2007;
226:
161
-168.
[PubMed]
.
-
53.
Festa
A
, D'Agostino
R Jr
, Williams
K
, Karter
AJ
, Mayer-Davis
EJ
, Tracy
RP
and Haffner
SM.
The relation of body fat mass and distribution to markers of chronic inflammation.
Int J Obes Relat Metab Disord.
2001;
25:
1407
-1415.
[PubMed]
.
-
54.
Pou
KM
, Massaro
JM
, Hoffmann
U
, Vasan
RS
, Maurovich-Horvat
P
, Larson
MG
, Keaney
JF Jr
, Meigs
JB
, Lipinska
I
, Kathiresan
S
, Murabito
JM
, O'Donnell
C J
and Benjamin
EJ.
Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress. The Framingham Heart Study.
Circulation.
2007;
116:
1234
-1241.
[PubMed]
.
-
55.
Kirkwood
TB
Understanding the odd science of aging.
Cell.
2005;
120:
437
-447.
[PubMed]
.
-
56.
Guarente
L
Sirtuins as potential targets for metabolic syndrome.
Nature.
2006;
444:
868
-874.
[PubMed]
.
-
57.
Libert
S
, Zwiener
J
, Chu
X
, Vanvoorhies
W
, Roman
G
and Pletcher
SD.
Regulation of Drosophila life span by olfaction and food-derived odors.
Science.
2007;
315:
1133
-1137.
[PubMed]
.
-
58.
Guarente
L
Mitochondria - a nexus for aging, calorie restriction, and sirtuins.
Cell.
2008;
132:
171
-176.
[PubMed]
.
-
59.
Burcham
PC
, Kaminskas
LM
, Fontaine
FR
, Petersen
DR
and Pyke
SM.
Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products.
Toxicology.
2002;
181-182:
229
-236.
[PubMed]
.
-
60.
Guiotto
A
, Calderan
A
, Ruzza
P
, Osler
A
, Rubini
C
, Jo
DG
, Mattson
MP
and Borin
G.
Synthesis and evaluation of neuroprotective α,β-unsaturated aldehyde scavenger histidylcontaining analogues of carnosine.
J Medicinal Chem.
2005;
48:
6156
-6161.
.
-
61.
Burcham
PC
and Pyke
SM.
Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection.
Mol Pharmacol.
2006;
69:
1056
-1065.
[PubMed]
.
-
62.
Tang
SC
, Arumugam
TV
, Cutler
RG
, Jo
DG
, Magnus
T
, Chan
SL
, Mughal
MR
, Telljohann
RS
, Nassar
M
, Ouyang
X
, Calderan
A
, Ruzza
P
and Guiotto
A.
Neuroprotective actions of a histidine analogue in models of ischemic stroke.
J Neurochem.
2007;
101:
729
-736.
[PubMed]
.
-
63.
Aldini
G
, Dalle-Donne
I
, Colombo
R
, Maffei
Facino R
, Milzani
A
and Carini
M.
Lipoxidation-derived reactive carbonyl species as potential drug targets in preventing protein carbonylation and related cellular dysfunction.
ChemMedChem.
2006;
1:
1045
-1058.
[PubMed]
.
-
64.
Galvani
S
, Coatrieux
C
, Elbaz
M
, Grazide
MH
, Jean
Claude T
, Parini
A
, Uchida
K
, Kamar
N
, Rostaing
L
, Baltas
M
, Salvayre
R
and Negre-Salvayre
A.
Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives.
Free Radic Biol Med.
2008;
45:
1457
-1467.
[PubMed]
.
-
65.
Virtue
S
and Vidal-Puig
A.
It's not how fat you are, it's what you do with it that counts.
PLoS Biol.
2008;
6:
e237
[PubMed]
.
-
66.
Berniakovich
I
, Trinei
M
, Stendardo
M
, Migliaccio
E
, Minucci
S
, Bernardi
P
, Pelicci
PG
and Giorgio
M.
p66Shc-generated oxidative signal promotes fat accumulation.
J Biol Chem.
2008;
283:
34283
-34293.
[PubMed]
.
-
67.
Di Gregorio
GB
, Yao-Borengasser
A
, Rasouli
N
, Varma
V
, Lu
T
, Miles
LM
, Ranganathan
G
, Peterson
CA
, McGehee
RE
and Kern
PA.
Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone.
Diabetes.
2005;
54:
2305
-2313.
[PubMed]
.
-
68.
Scherer
PE
Adipose tissue: from lipid storage compartment to endocrine organ.
Diabetes.
2006;
55:
1537
-1545.
[PubMed]
.
-
69.
Rasouli
N
, Molavi
B
, Elbein
SC
and Kern
PA.
Ectopic fat accumulation and metabolic syndrome.
Diabetes Obes Metab.
2007;
9:
1
-10.
[PubMed]
.
-
70.
Wang
MY
, Grayburn
P
, Chen
S
, Ravazzola
M
, Orci
L
and Unger
RH.
Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome.
Proc Natl Acad Sci USA.
2008;
105:
6139
-6144.
[PubMed]
.
-
71.
Kim
JY
, van
de Wall E
, Laplante
M
, Azzara
A
, Trujillo
ME
, Hofmann
SM
, Schraw
T
, Durand
JL
, Li
H
, Li
G
, Jelicks
LA
, Mehler
MF
and Hui
DY.
Obesity-associated improvements in metabolic profile through expansion of adipose tissue.
J Clin Invest.
2007;
117:
2621
-2637.
[PubMed]
.
-
72.
Hulbert
AJ
On the importance of fatty acid composition of membranes for aging.
J Theor Biol.
2005;
234:
277
-288.
[PubMed]
.
-
73.
Barja
G
Free radicals and aging.
Trends Neurosci.
2004;
27:
595
-600.
[PubMed]
.
-
74.
Pamplona
R
, Barja
G
and Portero-Otin
M.
Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation.
Ann NY Acad Sci.
2002;
959:
475
-490.
[PubMed]
.
-
75.
Sanz
A
, Pamplona
R
and Barja
G.
Is the mitochondrial free radical theory of aging intact.
Antioxid Redox Signal.
2006;
8:
582
-599.
[PubMed]
.
-
76.
Hulbert
AJ
, Faulks
SC
, Harper
JM
, Miller
RA
and Buffenstein
R.
Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes.
Mech Ageing Dev.
2006;
127:
653
-657.
[PubMed]
.
-
77.
Hulbert
AJ
, Pamplona
R
, Buffenstein
R
and Buttemer
WA.
Life and death: metabolic rate, membrane composition, and life span of animals.
Physiol Rev.
2007;
87:
1175
-1213.
[PubMed]
.
-
78.
Mukhopadhyay
A
, Deplancke
B
, Walhout
AJM
and Tissenbaum
HA.
C. elegans tubby regulates life span and fat storage by two independent mechanisms.
Cell Metabol.
2005;
2:
35
-42.
.
-
79.
Riddle
DL
Wood WB.
The dauer larva
Cold Spring Harbor, NY
Cold Spring Harbor Laboratory Press
393
-412.
The nematode C elegans.
1988;
.
-
80.
Garigan
D
, Hsu
AL
, Fraser
AG
, Kamath
RS
, Ahringer
J
and Kenyon
C.
Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation.
Genetics.
2002;
161:
1101
-1112.
[PubMed]
.
-
81.
Fei
YJ
, Liu
JC
, Inoue
K
, Zhuang
L
, Miyake
K
, Miyauchi
S
and Ganapathy
V.
Relevance of NAC-2, an Na+-coupled citrate transporter, to life span, body size and fat content in Caenorhabditis elegans.
Biochem J.
2004;
379:
191
-198.
[PubMed]
.
-
82.
Szewczyk
NJ
, Udranszky
IA
, Kozak
E
, Sunga
J
, Kim
SK
, Jacobson
LA
and Conley
CA.
Delayed development and lifespan extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction.
J Exp Biol.
2006;
209:
4129
-4139.
[PubMed]
.
-
83.
Wang
MC
, O'Rourke
EJ
and Ruvkun
G.
Fat metabolism links germline stem cells and longevity in C. elegans.
Science.
2008;
322:
957
-960.
[PubMed]
.
-
84.
Brenner S. The genetics of Caenorhabditis elegans.
Genetics.
1974;
77:
71
-94.
[PubMed]
.
-
85.
Kamath
RS
, Fraser
AG
, Dong
Y
, Poulin
G
, Durbin
R
, Gotta
M
, Kanapin
A
, Le
Bot N
, Moreno
S
, Sohrmann
M
, Welchman
DP
, Zipperlen
P
and Ahringer
J.
Systematic functional analysis of the Caenorhabditis elegans genome using RNAi.
Nature.
2003;
421:
231
-237.
[PubMed]
.
-
86.
Kamath
RS
and Ahringer
J.
Genome-wide RNAi screening in Caenorhabditis elegans.
Methods.
2003;
30:
313
-321.
[PubMed]
.
-
87.
Talreja
P
, Kleene
NK
, Pickens
WL
, Wang
TF
and Kasting
GB.
Visualization of the lipid barrier and measurement of lipid pathlength in human stratum corneum.
AAPS PharmSci.
2001;
3:
E13
[PubMed]
.
-
88.
Ogg
S
and Ruvkun
G.
The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway.
Mol Cell.
1998;
2:
887
-893.
[PubMed]
.
-
89.
Alin
P
, Danielson
UH
and Mannervik
B.
4-Hydroxyalk-2-enals are substrates for glutathione transferase.
FEBS Lett.
1985;
179:
267
-270.
[PubMed]
.
-
90.
Bunting
KD
, Lindahl
R
and Townsend
AJ.
Oxazaphosphorine-specific resistance in human MCF-7 breast carcinoma cell lines expressing transfected rat class 3 aldehyde dehydrogenase.
J Biol Chem.
1994;
269:
23197
-23203.
[PubMed]
.
-
91.
Möllering
H
and Gruber
W.
Determination of citrate with citrate lyase.
Anal Biochem.
1966;
17:
369
-376.
[PubMed]
.
-
92.
Lazzarino
G
, Amorini
AM
, Fazzina
G
, Vagnozzi
R
, Signoretti
S
, Donzelli
S
, Di Stasio
E
, Giardina
B
and Tavazzi
B.
Single-sample preparation for simultaneous cellular redox and energy state determination.
Anal Biochem.
2003;
322:
51
-59.
[PubMed]
.
-
93.
Satoh
K
, Yamada
S
, Koike
Y
, Igarashi
Y
, Toyokuni
S
, Kumano
T
, Takahata
T
, Hayakari
M
, Tsuchida
S
and Uchida
K.
A 1-hour enzyme-linked immunosorbent assay for quantitation of acrolein- and hydroxynonenal-modified proteins by epitope-bound casein matrix method.
Anal Biochem.
1999;
270:
323
-328.
[PubMed]
.
-
94.
Gree
R
, Tourbah
H
and Carrie
R.
Fumaraldehyde monodimethyl acetal: an easily accessible and versatile intermediate.
Tetrahedron Lett.
1986;
27:
4983
-4986.
.
-
95.
Chandra
A
and Srivastava
SK.
A synthesis of 4-hydroxy-2-trans-nonenal and 4-(3H) 4-hydroxy-2-trans-nonenal.
Lipids.
1997;
32:
779
-782.
[PubMed]
.