Abstract
The nutrient-sensing target of rapamycin (TOR) pathway appears to have a conserved role in regulating life span. This signaling network is complex, with many downstream physiological outputs, and thus the mechanisms underlying its age-related effects have not been elucidated fully. We demonstrated previously that reduced TOR signaling (intor1Δ strains) extends yeast chronological life span (CLS) by increasing mitochondrial oxygen consumption, in part, by up-regulating translation of mtDNA-encoded oxidative phosphorylation (OXPHOS) subunits. Here, we have examined in greater detail how TOR signaling influences mitochondrial function and CLS and the role of the Sch9p kinase in the TOR-mitochondria pathway. As is the case for oxygen consumption, mitochondrial translation is elevated in tor1Δ strains only during active growth and early stationary phase growth points. This is accompanied by a corresponding increase in the abundance of both mtDNA-encoded and nucleus-encoded OXPHOS subunits per mitochondrial mass. However, this increased OXPHOS complex density is not associated with more mitochondria/cell or cellular ATP and leads to an overall decrease in membrane potential, suggesting that TOR signaling may influence respiration uncoupling. Finally, we document that the Sch9p kinase is a key downstream effector of OXPHOS, ROS and CLS in the TOR-mitochondria pathway. Altogether, our results demonstrate that TOR signaling has a global role in regulating mitochondrial proteome dynamics and function that is important for its role in aging and provide compelling evidence for involvement of a "mitochondrial pre-conditioning" effect in CLS determination.
Introduction
How and why we age has long been a
fascination of humans. In addition to being of intrinsic philosophical,
evolutionary and biological interest, determining the molecular and cellular
mechanisms underlying the aging process is relevant to understanding
age-related pathology that ultimately limits human life and health span. Model
organism studies have been instrumental in understanding
aging, with many conserved pathways and
factors having been identified in files, worms and yeast (and other organisms)
that have physiological and pathological relevance in humans [1]. One general
area that has been implicated strongly in aging and life span determination is
nutrient availability/sensing. For example, dietary (i.e. caloric) restriction
extends life span and ameliorates many of the age-associated declines in
cellular function in virtually all organisms examined to date [2].
One
major consequence of changing nutrient availability/sensing is alternation of
cellular metabolism and mitochondrial respiration. Life span extension by
caloric restriction, for instance, usually involves enhanced mitochondrial
activity [2,3]. While best known for providing ATP via oxidative
phosphorylation (OXPHOS), mitochondria are a major crossroads for anabolic and
catabolic metabolism, as well as many other critical cellular functions such as
apoptosis, signal transduction, and ion homeostasis [4]. Mitochondria also
contain a DNA genome (mitochondrial DNA; mtDNA) that harbors a set of genes
involved in OXPHOS and requires dedicated machinery for organellar DNA
replication and gene expression that is encoded primarily by genes in the
nucleus (e.g. mitochondrial DNA and RNA polymerase, ribosomes, transcription
and translation factors, etc) [5,6]. Mitochondria also generate reactive
oxygen species (ROS) as byproducts of the electron transport process, which is
a major way they are thought to contribute to the aging process. For example,
the "mitochondrial theory of aging", which builds on Harman's "free-radical"
theory, posits that ROS from mitochondrial respiration damage cellular
components, including mtDNA, and lead to declines in cell, tissue and
organismal function over time [7,8]. As ROS are also signaling molecules,
altered signal transduction is another potential contributor to aging
phenotypes due to mitochondrial dysfunction [9]. While the mechanisms through
which altered respiration affects life span are complex and have not been
defined fully, differential ROS production is likely involved. For example,
aberrant respiration due to defective RAS signaling [10], pharmacological
inhibition [11], or imbalanced translation of mtDNA-encoded OXPHOS subunits [9]
elevates cellular ROS and severely curtails yeast chronological life span
(CLS). Conversely, mild uncoupling of mitochondrial respiration extends yeast
CLS and decreases ROS [11].
Several kinase pathways serve as
physiological switches in response to nutrient availability. For example, the
conserved target of rapamycin (TOR) signaling pathway controls growth by
positively regulating the processes of ribosome biogenesis and cytoplasmic
translation when preferred nutrient supplies are available. In yeast, the TOR
pathway also negatively regulates stress response genes, autophagy, and usage
of alternate carbon and nitrogen sources [12]. Thus, when nutrients are
limiting, TOR activity is reduced, energy is conserved (by shutting down
expensive growth-promoting pathways) and diverted to provide stress resistance
and access to alternate energy stores. The TOR
kinase forms two multi-protein complexes, TORC1 and
TORC2, with TORC1 functioning as the nutrient sensor [12]. In yeast, there are
two TOR kinase genes TOR1 and TOR2. Both Tor1p and Tor2p can
function in the TORC1 complex, but only Tor2p can function in the TORC2
complex. Thus, deletion of TOR1 results in reduced TORC1 signaling, but
is not lethal. This is because Tor2p can partially cover the loss of Tor1p in
TORC1, while still also functioning in TORC2. In contrast, deletion of TOR2 is lethal [13]. Reduced TORC1 signaling extends life span in a number of model
organisms including yeast (S. cerevisiae), worms (C. elegans) and
flies (D. melanogaster) [14-17]. We recently reported that a major
mechanism underlying this phenotype in yeast is enhanced mitochondrial
respiration driven, at least in part, by increased translation of mtDNA-encoded
OXPHOS subunits [18]. In that study, we speculated that the extension of CLS by
reduced TOR signaling involves an increase in the number of OXPHOS complexes
per organelle that increases oxygen consumption, decreases ROS production in
stationary phase, and thereby limits damage to cellular components. However,
since mtDNA encodes only minority of the OXPHOS complex subunits (i.e. of the
~80 OXPHOS subunits only seven in yeast and thirteen in mammals are encoded by
mtDNA) and mitochondria contain >1,000 proteins (encoded by nuclear genes
and imported into the organelle), the possibility that TOR signaling regulates
mitochondria in a more global fashion is likely. In fact, TOR-dependent
changes in the mitochondrial proteome have been documented in human Jurkat T
cells [19].
Sch9p
belongs to the AGC family of kinases and is a key downstream target of TORC1
signaling in yeast. For example, Sch9p is a functional ortholog of ribosomal
protein S6 kinase, a key mediator of mTOR signaling in mammalian cells [20].
TORC1 directly phosphorylates Sch9p at multiple sites, which is important for
modulating cytoplasmic translation and cell cycle progression. Sch9p is also a
negative regulator of both chronological and replicative aging [14,21] and has
recently been shown to similarly regulate mitochondrial respiration [22]. In
fact, like deletion of TOR1, deletion of SCH9 extends yeast CLS
in a respiration-dependent fashion, suggesting that Sch9p could be a downstream
mediator of TOR-dependent mitochondrial OXPHOS regulation in this regard. In
the current study, we have examined in greater mechanistic detail how the yeast
TOR pathway influences mitochondrial gene expression, OXPHOS activity, and
proteome composition, and the role of the Sch9p kinase as a downstream mediator
of its effects on mitochondria.
Results
Reduced
TOR signaling globally increases mitochondrial translation and results in a
greater number of OXPHOS complexes per organelle
We
demonstrated previously that reduced TOR signaling (in tor1 null yeast
strains; tor1Δ) results in increased mitochondrial translation
rates, oxygen consumption, and life span [18]. This is accompanied by a
corresponding increase in the steady-state levels of mtDNA-encoded OXPHOS
subunits. However, whether there is global up-regulation of mitochondrial
translation was not addressed in that study and only a single, late culture
growth point was analyzed. To better understand the mitochondrial translation
response to reduced TOR signaling, we labeled all mtDNA-encoded subunits at
three growth points and visualized the individual products by autoradiography
of SDS-PAGE gels. Compared to wild-type strains, we observed global
up-regulation of mitochondrial translation products in log-phase and early
stationary phase (day 1) cultures in tor1D strains (Figure 1). One day later in stationary phase
(day 2) the wild-type and tor1Δ strains showed
similar rates of mitochondrial translation, due to an increase in the rate in
the wild-type strains (Figure 1). These results mirrored closely our previously
published results on oxygen consumption as a function of growth state and
demonstrate that the major differences in mitochondrial function in these
strains are manifest during growth and early stationary phase, which is when
TOR signaling is at its highest in wild-type strains.
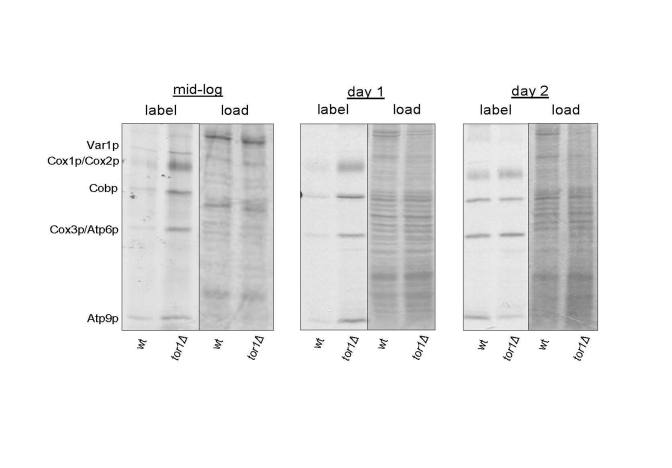
Figure 1. Elevated mitochondrial translation rates in tor1Δ strains during the exponential and early stationary growth phases. Results of an in vivo-labeling experiment in which
the mtDNA-encoded gene products are labeled specifically and visualized by autoradiography
after separation by SDS-PAGE (see Materials and Methods). Wild-type (wt) and tor1 null
(tor1Δ) strains labeled at mid-log, early stationary (day 1) and later stationary (day 2) are shown.
The left-half panel under each time point is the autoradiogram showing the labeled mitochondrial
gene products (with each product indicated on the left) and the right-hand panel is the respective
Coomassie blue-stained gel as a control for total protein loading.
The observed increase in mitochondrial
translation in tor1D strains prompted us to
examine additional mitochondrial parameters. Here, we focused on mid-log growth
points, where the largest differences in mitochondrial translation and oxygen
consumption are observed. First, consistent with the increase in mitochondrial
translation, there was an increase in the steady-state levels of mtDNA-encoded
OXPHOS subunits (3-12 fold) per mitochondrial mass as judged by western
blotting of Cox1p, Cox2p and Cox3p in mitochondrial extracts (Figure 2A). This
was accompanied by an increase in the Cox4p OXPHOS subunit (2.2 fold), but not
of porin, both of which are encoded by nuclear genes (Figure 2A). This result
suggested to us that the OXPHOS machinery was up-regulated more or less
specifically and that an overall increase in mitochondrial biogenesis was not
occurring. To test this hypothesis, we transformed the strains with a plasmid
encoding a mitochondria-targeted GFP protein and measured mitochondrial content
by FACS, as well as determined mtDNA copy number, amounts of which usually
correlate with mitochondrial abundance. No significant differences in
mitochondrial mass (Figure 2B) or mtDNA (Figure 2C) were observed between the
wild-type and tor1D strains. There also were no
obvious differences in mitochondrial distribution or morphology observed by
fluorescence microscopy of the GPF-containing strains (data not shown).
Altogether, these data indicate that there is an increase in the number of OXPHOS
complexes per organelle mass in tor1D strains, as opposed to a global up-regulation of the
amount of mitochondria per cell. However, despite the fact there is increased
mitochondrial OXPHOS components and oxygen consumption, there was a reduction
in mitochondrial membrane potential (Figure 2D) and no significant difference
in total cellular ATP in tor1D strains (data not shown).
To
better understand how reduced TOR signaling dynamically effects respiration, we
used the TOR kinase inhibitor rapamycin under a variety of conditions. Addition
of rapamycin to a wild-type culture from the beginning of growth resulted in a
significant and sustained increase in mitochondrial oxygen consumption
(Supplementary Figure 1A), similar to that observed in tor1D strains.
However, rapamycin greatly inhibited the growth rate of these strains (data not
shown). In contrast, adding rapamycin at a later point during growth (after the
strains reached OD ~1.0) only increased oxygen consumption by ~30%
(Supplementary Figure 1B). This increase required the presence of the drug for 2-4 hours, was
sustained for at least 30 hours (Supplementary Figure 1B), and depended on both cytoplasmic
and mitochondrial translation (i.e. was inhibited by addition of either
cycloheximide or chloramphenicol; data not shown).
Reduced
TOR signaling increases the steady-state levels of mitochondrial transcripts
Given
that the overall rates of mitochondrial translation were higher in tor1D strains, but mtDNA copy number was not, led us to
investigate the whether there were changes in steady-state levels of
mitochondrial transcripts that might indicate a mtDNA transcriptional response.
Northern blots of three mitochondrial mRNA transcripts revealed that there is a
1.5- to 2.1-fold increase in tor1Δ strains (using
25S rRNA as a loading control; Figure 3). Similar changes were observed in the
14S rRNA (data not shown). These data indicate that there is a moderate
increase in mitochondrial transcripts in tor1D strains, but that this is unlikely to be the primary
driving force behind the significantly greater rates of mitochondrial
translation and OXPHOS complex abundance observed.
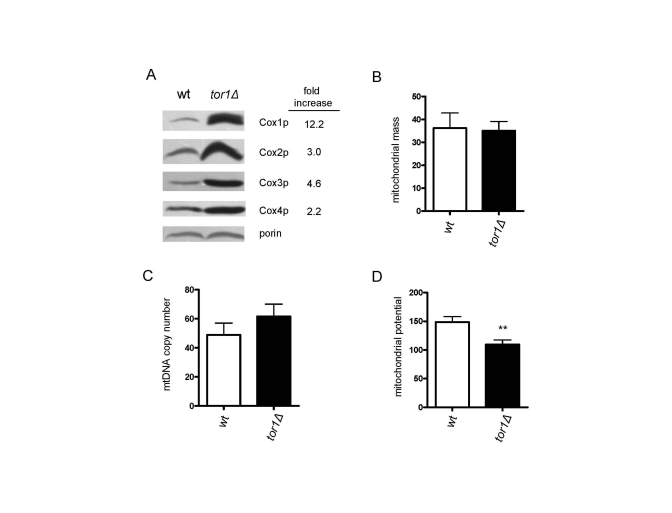
Figure 2. Reduced TOR signaling increases the number of mitochondrial OXPHOS complexes per organelle, as opposed to the number of mitochondria/cell. Comparative analysis of four mitochondria-related
parameters in wild-type (wt) and tor1Δ strains is shown. (A)
Western blot analysis of four OXPHOS subunits (Cox1p-4p) and porin (as a
mitochondrial normalization control). Fifty μg of mitochondrial extract was loaded in each lane. The fold
difference between wt and tor1Δ normalized to the porin signal is shown on the right. (B)
Mitochondrial mass as estimated by the amount of mitochondrial-GFP signal
determined by FACS (see Materials and Methods). (C) mtDNA copy
number determined by real-time PCR (measured as the ratio of the
mitochondrial gene target COX1 relative to the nuclear gene target ACT1). (D) Mitochondrial membrane potential determined by DiOC6 staining and FACS analysis. In B-D means of at least three biological
replicates +/- one standard deviation are graphed (** represents a p-value
from a student t-test that is <0.01).
Global up-regulation of OXPHOS-related proteins in tor1Δ mitochondria revealed by 2D-DIGE
To gain a better understanding of how reduced TOR
signaling affects mitochondria, we have begun to characterize changes in the
mitochondrial proteome in tor1D strains by
two-dimensional, differential gel electrophoresis (2D-DIGE), coupled to mass
spectro-metry-based identification of differentially regulated proteins. Given
that we observed an increase in OXPHOS subunits/mitochondrial mass by western
blot (Figure 2A), we have focused initially on those proteins that were
up-regulated by 2-fold or greater in mito-chondria from tor1Δ strains (see Materials and Methods). Of the 26 up-regulated spots
picked and analyzed based on this 2-fold cutoff, we have unambiguously
identified eleven proteins that are at higher steady state-levels in
mitochondria purified from tor1D strains in the mid-log
growth phase (Table 1). In addition to Cox4p, which we had already documented
as increased by western blot (Figure 2A), we identified five other OXPHOS
components: Cox13p (another subunit of Complex IV), Qcr7 (subunit of Complex
III), and Atp2p, Atp5p and Atp7p (subunits of Complex V/ATP synthase). In
addition to OXPHOS components, we have thus far identified five other proteins
that are up-regulated in mitochondria from tor1D strains (Table 1).
Three of these (Dld2p, Gcv3p, and Ilv6p) are involved
in various aspects of metabolism, one (Om45p) is
an abundant outer mitochondrial membrane protein of unknown function, and the
final one (Yhb1p) is involved in nitric oxide detoxification. Altogether, these
data solidify our contention that that there is global up-regulation of OXPHOS
machinery/organelle in response to reduced TOR signaling, but also indicate
that TOR activity impacts mitochondrial proteome composition in other
interesting ways. Furthermore, in the case of Gcv1p and Ilv6p, the spots
identified of wild-type differ in molecular weight and/or PI from those of tor1Δ (data not shown), suggesting that TOR regulates expression and/or
processing of these proteins in a unique manner.
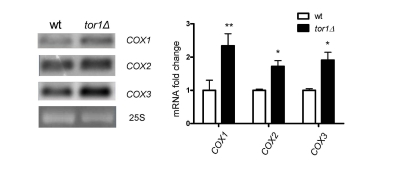
Figure 3. Increase of mitochondrial transcript abundance in tor1Δ strains.
Northern analysis of the mtDNA-encoded mRNA transcripts COX1-COX3 from wild-type (wt) and tor1Δ
strains is shown, along with ethidium bromide-stained nuclear 25S rRNA as a loading control.
Graphed on the right is the mean fold difference in COX1, COX2, and COX3 abundance normalized
to 25S rRNA +/- one standard deviation (* designates a p-value <0.05 and ** designates a p-value
<0.01 based on a student's t-test).
Balanced expression of mitochondrial OXPHOS components is
required for extension of chronological lifespan mediated by reduced TOR signaling
We
previously documented that strains with imbalanced expression of mtDNA-encoded
OXPHOS subunits have reduced chronological life span (CLS) [9]. One strain
(GS129), in particular, has a severely curtailed CLS due to
a point mutation in the amino-terminal domain of mtRNA polymerase (Rpo41p) that
results in increased ROS [9]. Given that reduced TOR signaling
(due to TOR1 deletion) increases CLS, in part by increasing the rate of mitochondrial
translation [18], we used the GS129 strain background to address the
requirement for balanced
mtDNA expression in this regard. Deletion of TOR1 in the GS129
background resulted in an increase in translation of most mtDNA-encoded
products to a degree that exceeded that in the isogenic wild-type strain GS122,
but less than that observed in the isogenic wild-type tor1D strain (GS122 tor1Δ) (Figure 4A). However, unlike in the wild-type strain, there was no
significant increase in Cox1p translation when TOR1 was deleted in the
GS129 background (Figure 4A). In other words, translation was increased in the
GS129 strain in response to reduced TOR signaling, but not in a balanced
manner. Analysis of CLS in these strains revealed that deletion of TOR1 extended life span in the wild-type (GS122) background as expected, but did not
significantly increase CLS in the "imbalanced" GS129 strain (Figure 4B). These
data indicate that extension of life span by
reduced TOR signaling requires balanced up-regulation of OXPHOS components
encoded by mtDNA.
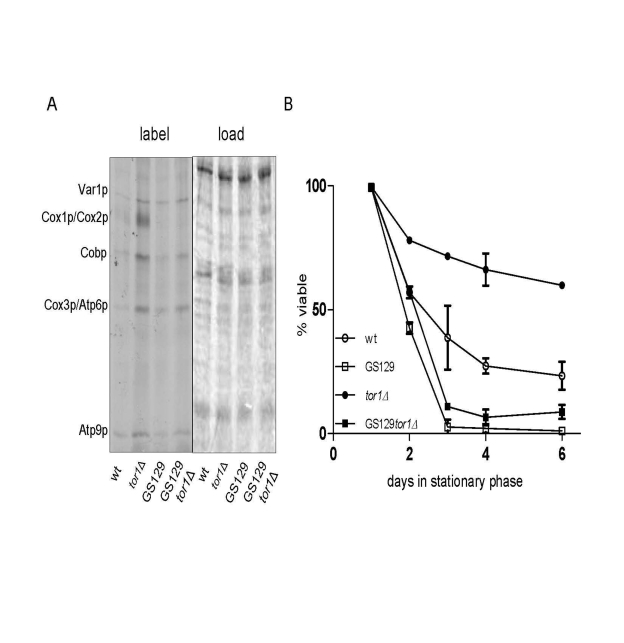
Figure 4. Reduced TOR signaling does not rescue chronological life span in the short-lived GS129 strain with imbalanced mitochondrial translation. (A) Results of mitochondrial translation assays are shown as described
in Figure 1. The strains analyzed are GS122 (wt with regard to RPO41) and GS129
(containing the rpo41-R129D point mutation) in which the TOR1 gene was (tor1Δ)
or was not (wt) disrupted (see Materials and Methods).
(B) Chronological life span curves of the same strains in A. are shown.
Three independent colo-nies of each strain were analyzed and the mean % viability +/-
one standard deviation is plotted according to the key in the lower left corner.
SCH9 is a downstream target of TOR signaling in the regulation of mitochondrial function
Recently, deletion of SCH9 was also shown to increase expression of mitochondrial
OXPHOS genes and mitochondrial respiration [22]. Given that these
mitochon-drial phenotypes are similar to those we have documented here and
previously in tor1D strains, we tested the
hypothesis that SCH9 is downstream of TOR1 with regard to
mitochondrial regulation by simultaneously analyzing isogenic single (tor1D or sch9Δ) and double (tor1Δ sch9Δ) knock-out
strains. As reported previously [18], we observed an increase in mitochondrial
oxygen consumption in the sch9Δ strain that was
similar in magnitude (2-fold) to the
increase observed in the isogenic tor1Δ strain (Figure 5A). However,
this increase was not enhanced further in
the tor1Δ sch9Δ double-mutant
strain (Figure 5A), consistent with these genes being in the same pathway with regard to mitochondrial
respiration. Similar results were obtained for mitochondrial translation rates
(Figure 5B) and steady-state levels of nucleus and mtDNA-encoded OXPHOS
proteins (Figure 5C). However, the sch9Δ single mutant had a greater effect than the tor1Δ single mutant on these latter three parameters, and there was no
synergistic effect observed in the double-mutant strains (Figures 5A and 5B).
The fact that the double-mutant strain more closely resembled the sch9Δ strain
is most consistent with SCH9 being downstream of TOR1 in this pathway controlling
mitochondrial translation and respiration. This was evidenced further by the fact
that addition of rapamycin to wild-type strains caused an increase
in mitochondrial translation that was greater in magnitude to that observed in
the tor1Δstrain
(Figure 5B). That is, rapamycin or SCH9 deletion appears to represent a
more complete down-regulation of TOR signaling than deletion of TOR1.
Finally, comparison of the actin and porin ratio (an indicator of mitochondrial
abundance) in the single and double mutant strains (Figure 5C) confirmed that,
as was the case for tor1Δ, there was no
significant increase in overall mitochondrial biogenesis in the sch9Δ and tor1Δ sch9Δ strains, but rather an increase in the number of OXPHOS complexes per
organelle mass, again placing these two genes in the same pathway with regard
to mitochondrial function.
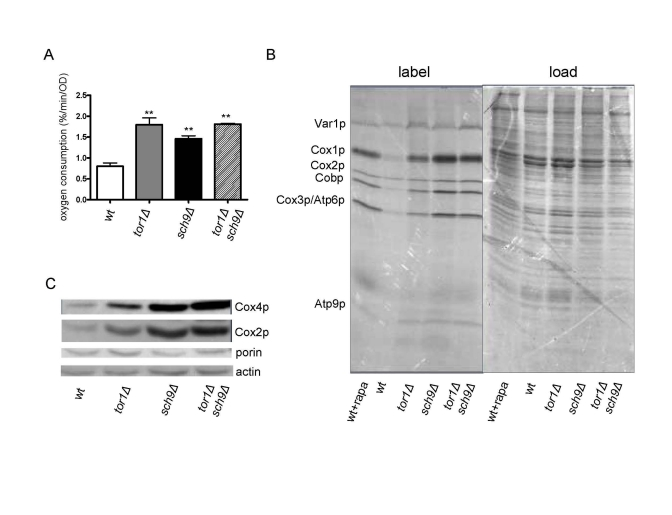
Figure 5. Sch9p mediates TOR-dependent increases in mitochondrial function. Comparative analysisof
mitochondria-related parameters in wild-type (wt), tor1Δ, sch9Δ and sch9Δtor1Δ strains in the DBY2006 genetic background.
(A) Mitochondrial oxygen consumption. (B) Mitochondrial
translation as described in Figure 1. (C) Western blot of the
Cox1p, Cox4p, porin and actin OXPHOS components in the four strains using
100 μg of whole cell extract in each
lane. We use the ratio of porin to actin as one measure of mitochondrial
abundance per cell (which is virtually the same between the strains) and
the ratio of Cox subunits to porin to demonstrate their specific increase
per mitochondrial mass.
Table 1. Mitochondrial Proteins Identified as Up-regulated in tor1Δ Yeast Strains by 2D-DIGE.
|
Protein
|
ID
|
Protein
Function
|
Expression
Ratio tor1/wt
|
| OXPHOS Components | |
|
Atp2p
|
gi|151945186
|
F1F0
ATP synthase beta subunit
|
2.09
|
|
Atp5p
|
gi|6320504
|
Subunit
5 of the stator stalk of mitochondrial F1F0 ATP synthase
|
2.51
|
|
Atp7p
|
gi|151941529
|
F1F0
ATP synthase subunit d
|
2.48
|
|
Cox13p
|
gi|6321247
|
Subunit
VIa of cytochrome c oxidase
|
3.33
|
|
Cox4p
|
gi|6321251
|
Subunit
IV of cytochrome c oxidase
|
2.19
|
|
Qcr7p
|
gi|6320738
|
Subunit
7 of the ubiquinol cytochrome-c reductase complex
|
2.20
|
| Outer
Membrane Protein | |
|
Om45p
|
gi|6322055
|
Protein
of unknown function, major constituent of the mitochondrial outer membrane
|
2.33
|
| Metabolic
Enzymes | |
|
Dld2p
|
gi|51830216
|
D-lactate
dehydrogenase, located in the mitochondrial matrix
|
2.51
|
|
Gcv3p*
|
gi|595540
|
H-protein
subunit of the glycine cleavage system
|
2.60
|
|
Ilv6p*
|
gi|6319837
|
Regulatory
subunit of acetolactate synthase, which catalyzes the first step of
branched-chain amino acid biosynthesis
|
2.53
|
| Detoxification
Enzyme | |
|
Yhb1p
|
gi|6321673
|
Nitric
oxide oxidoreductase, flavohemoglobin involved in nitric oxide detoxification
|
2.45
|
SCH9 is downstream of TOR1 in the regulation of chronological life span
We previously implicated reduced ROS in
stationary phase as a significant factor that increases the CLS of tor1Δ strains [18]. A similar reduction in ROS was also observed in sch9Δ and tor1Δ sch9Δ strains (Figure 6A), again consistent with SCH9 working in the
same genetic pathway as TOR1 with regard to mitochondria-derived ROS.
Finally, as was the case for mitochondrial translation and OXPHOS complex
abundance, we found that deletion of SCH9 increased CLS to a greater
degree than deletion of TOR1, but that there was no further increase in
CLS in the tor1Δ sch9Δ double mutant
strain (Figure 6B). Altogether, these data solidify the connections between
mitochondrial OXPHOS, ROS and CLS and demonstrate that Sch9p is a key downstream factor that mediates the effects
of TOR signaling on mitochondrial function and yeast aging.
Discussion
This
study provides significant new insight into the mechanism through which TOR
signaling controls mitochondrial function to influence yeast CLS and elucidates
which arm of the TORC1 pathway is involved. The primary conclusions we draw
from the results obtained are that 1) reduced TORC1 signaling (via deletion of
the TOR1 gene) increases respiration primarily through up-regulation of
the number of OXPHOS complexes/organelle, not by increasing overall
mitochondrial biogenesis, 2) the up-regulation of OXPHOS complexes involves
both mtDNA-encoded and nucleus-encoded subunits and, in terms of mtDNA
expression, occurs primarily via translational regulation, 3) in addition to
its effects on OXPHOS complex abundance, TOR signaling controls other aspects
of mitochondrial proteome dynamics, 4) TOR-dependent changes in mitochondrial
function and CLS are mediated by the downstream Sch9p kinase, and 5) it is
TOR-dependent alterations of mitochondrial function in the exponential and/or
post-diauxic-early stationary growth phases that subsequently impact late
stationary-phase survival and extend CLS, which suggests a role of
"mitochondrial pre-conditioning" on yeast aging. The basis of these conclusions
and additional interpretations are discussed below.
The
increase in cellular mitochondrial oxygen consumption (i.e. respiration) in
response to reduced TOR signaling reported herein (Figure 5A) and previously
[18] could occur by one of several mechanisms that are not mutually exclusive.
For example, it could be mediated by direct effects on the activity of existing
OXPHOS complexes, by increasing overall mitochondrial biogenesis (resulting in
more mitochondria/cell), or by increasing the number of OXPHOS complexes per
organelle. Our results demonstrate that increasing organelle OXPHOS complex
density is definitely one mechanism at play.
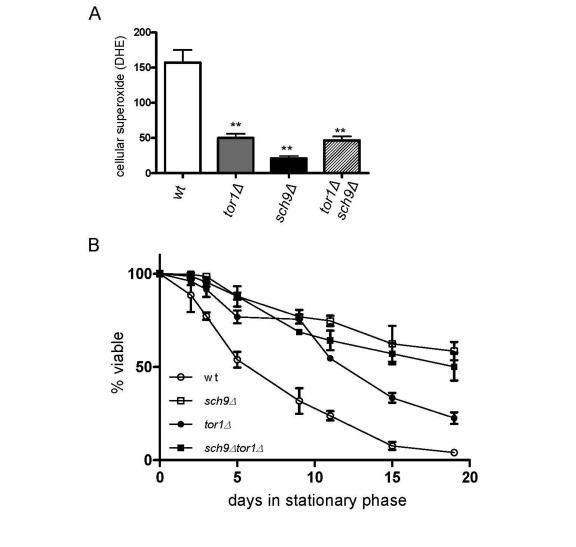
Figure 6. Sch9p is the downstream mediator of TOR-dependent decreases in ROS level and chronological life span extension. Analysis of cellular ROS and
chronological life span in the same strains shown in Figure 5. (A)
FACS analysis of day 2 stationary phase cells stained for cellular
superoxide using dihyroethidium (DHE) is shown. The mean fluorescence
intensity is plotted +/- one standard deviation (** represents a p-value
<0.01 according to a student's t-test). (B) Chronological life
span plotted as described in Figure 4B.
The
basis for this conclusion is that, in mitochondrial extracts, we observe
increased abundance of both nuclear and mitochondrial OXPHOS subunits, but not
other mitochondrial markers (e.g. porin; Figure 2A). This result was
substantiated by our initial 2D-DIGE proteomic analysis of highly purified
mitochondria from wild-type and tor1Δ strains,
in which we identified OXPHOS complex subunits (from three separate complexes)
as proteins that are in significantly higher abundance in mitochondria from tor1Δ cells (Table 1). Finally, there was no increase in overall
mitochondrial biogenesis as judged by mtDNA content (Figure 2C), labeling of
mitochondria with a GFP marker and analyzing them by FACS (Figure 2B), and
western blot comparisons of mitochondrial and cytoplasmic markers (Figure 5C).
While increased OXPHOS complex density is clearly occurring, we have not
eliminated the possibility that there are also TOR-dependent effects on the
enzymatic activity of the complexes that contribute to the increase in oxygen
consumption.
Our
2D-DIGE results are not entirely consistent with a recent proteomic study of
rapamycin-treated yeast cell [23], where fewer OXPHOS proteins were identified
as up-regulated. We found that addition of rapamycin during the growth phase
impacts mitochondrial oxygen consumption on a longer time scale and to a lesser
degree compared to adding rapamycin from the beginning of the growth experiment
(i.e. at inoculation; Supplementary Figure 1). The latter condition is in some
ways more similar to the tor1Δ strains
analyzed in this study in that, in this case, TOR signaling is reduced
throughout all stages of growth. Thus, differences in the timing and/or degree
of TOR inhibition may explain the different results obtained in the two
studies.
To
increase OXPHOS complex density as a means to increase mitochondrial oxygen
consumption is to our knowledge a unique mechanism of mitochondrial regulation
levied by the TOR pathway. We originally hypothesized that this would lead to
greater mitochondrial membrane potential due to the increase in electron
transport activity and perhaps also a higher cellular ATP. However, this was
not the case; there was instead a decrease in membrane potential in tor1Δ strains (Figure 2D) and no change in cellular ATP (data not shown).
Thus, in tor1Δ strains, there in an increase in electron transport
activity (i.e. oxygen consumption) and a decrease in mitochondrial membrane
potential, which equates to a mitochondrial network with overall lower energy
capacity on average. One potential explanation for this result is that reduced
TOR signaling is leading to an increase in uncoupled respiration. This would
lead to increased oxygen consumption in an attempt to maintain the membrane
potential in the face of the proton leak and an inability to simultaneously
increase ATP production. Since mild uncoupling also increases CLS [11], this
indeed may prove to be the mechanism through which TOR signaling influences
aging in yeast. Testing this hypothesis is a logical area of future
investigation, but certainly other explanations can be envisioned.
In order to affect an increase in OXPHOS
complexes per mitochondrion, the cell needs to increase the production and/or
stability of both mtDNA-encoded and nucleus-encoded OXPHOS subunits, while not
inducing a full mitochondrial biogenesis response. How reduced TOR signaling
accomplishes this remains to be determined, yet several insights are gleaned
from our results. First, we observe an increase in both mtDNA-encoded and
nucleus-encoded OXPHOS subunits (Figure 2A, Table 1), thus TOR signaling is
affecting both mitochondrial and nuclear gene expression simultaneously.
According to our results, this is occurring both at the mRNA level (Figure 3)
and at the translational level (Figure 1) in mitochondria, but not at the level
of protein stability to any obvious degree (Figure 5B, Supplementary Figure 2).
Since transcript-tion and translation are coupled in mitochondria [24-26],
these changes probably work together to mediate the increase in OXPHOS complex
abundance in tor1Δ strains. Although, the
translational control appears to contribute to a greater extent, given only
modest changes in mitochondrial transcript levels are observed. However, the
change in mitochondrial transcripts of tor1Δ strains might represent a reduction of glucose repression, which is
known to induce mitochondrial transcription [27,28] and mimic the effects of tor1Δ on respiration and CLS based on our previous study [18]. Interesting
in this regard is the key role of the Snf1p kinase in the glucose repression
phenomenon [29]. Snf1p is the yeast ortholog of mammalian AMP kinase, which
negatively regulates mTOR signaling in response to energy charge by activating
Tsc2, an inhibitor of mTORC1 [30]. Though a Tsc2 ortholog appears to be absent
in yeast, these correlations might suggest an evolutionarily conserved
regulatory framework that links glucose metabolism, TOR signaling,
mitochondrial gene expression and life span.
Whether
the increase in nuclear OXPHOS gene expression is mediated at the
transcriptional or post-transcriptional level remains to be determined, as does
the identity of the putative TOR-regulated mitochondrial factors that meditate
the increase in mitochondrial mRNA transcription/stability and translation of
mtDNA-encoded OXPHOS subunits. Certainly, nuclear transcription factors that
are known to be downstream of TORC1 [31], involved in nuclear-mitochondrial
signaling [32], or in glucose repression of mitochondrial function [33] are
obvious candidates to test with regard to the nuclear gene expression response.
And, with regard to TOR-dependent factors that regulate mitochondrial gene
expression directly, the mitochondrial transcription machinery, mitochondrial
ribosomes, or the various general and specific translational activators [5] are
likely candidates to consider in future studies. Furthermore, since our results
clearly implicate Sch9p as the key mediator of the TORC1-mitochondria-CLS
pathway (Figure 6B), searching for mitochondrial substrates of Sch9p as
potential downstream targets that execute changes in mitochondrial gene
expression and OXPHOS activity would likely be fruitful.
The
fact that up-regulation of mitochondrial oxygen consumption [18] and mitochondrial
translation (Figure 1) in tor1Δ strains occurs
only in log-phase and early stationary phase cultures (and not later in
stationary phase) strongly suggests that TOR-dependent mitochondrial changes
that occur early are responsible for the life span extension later in
stationary phase. The concept of early mitochondrial-related events effecting
life span has been promoted by others in aging studies in C. elegans [34,35] and is also consistent with the observation of Piper and colleagues
that previous conditioning of yeast to respiratory conditions extends CLS in
subsequent cultures [36]. While, at this point, the molecular explanation for
this "mitochondrial pre-conditioning" effect is not clear, we consider ROS
signaling as one potential model. This idea is attractive because the rate of production
of ROS from the mitochondrial electron transport chain is likely an accurate
reflection of mitochondrial OXPHOS activity and/or redox status that could be
used by cells as a retrograde signal to modulate nutrient-sensing pathways.
Although we have not observed a significant change in the steady-state level of
superoxide in log-phase tor1Δ cells (data not
shown), it is possible that other ROS species may be relevant or that the
steady-state measurements are not accurately predicting the rate of
mitochondrial ROS production. Alternatively, we observed up-regulation of Yhb1,
a nitric oxide detoxifying enzyme in tor1Δ mitochondria, but not Sod2p (data not shown; [18]). These results
might suggest a role for NO and/or other reactive nitrogen species as relevant.
Interesting in this regard, as is the case in tor1Δ cells (Table 1), Yhb1p localizes to mitochondria under anaerobic
condition [37]. This, coupled to our observation that hypoxic conditions bypass
the extension of CLS by TOR1 deletion [18] might suggest that reduced
TOR signaling and anaerobic conditions share a common route to impact life span
that may involve NO metabolism. Future studies along these and related lines,
as well as further characterization of TOR-dependent changes in the
mitochondrial proteome should be most revealing in terms of understanding how
the TOR-mitochondria axis controls aging and deciphering the complex
relationships between OXPHOS activity, ROS (and/or other reactive species),
nutrient sensing, and life span. This, in turn, may provide new inroads into
understanding and perhaps counteracting age-related pathology in humans.
Materials and Methods
Yeast strains.
Unless
otherwise stated, strains of the DBY2006 (MATa his3-Δ200 leu2-3,-112 ura3-52 trp1- Δ1 ade2-1)
background were used exclusively. The GS122 and GS129 strains are derivatives
of DBY2006 that have plasmid-borne RPO41 and rpo41-R129D alleles
covering a chromosomal disruption of the endogenous RPO41 gene and have
been described previously [24]. These strains were used for the experiments
presented in Figures 1 and 4. The TOR1 gene was disrupted in these
strains as described previously [18]. The SCH9 gene was disrupted using
a standard HIS3 knockout cassette [38]. Briefly, the HIS3 in
pRS313 was PCR amplified with primers
ACCACCGCTATTAGTCAGGACTTATATGCAATGGGCACAACAGGAATAACAAGATTGTACTGAGAGTGCAC (SCH9_LeftDel)
and CATCATTGATGTCC TCGTCCCCGTCATCATCGATGACATCTTCGTCTG GACTGTGCGGTATTTCACACCG (SCH9_RightDel).
Gel-purified amplicons were used to transform
wild-type and tor1Δ DBY2006. His+ transformants
were selected on his- plates and single colonies were picked and verified by
PCR. The mitochondrial GFP expressing yeast strains were generated by
transforming wild-type DBY2006 and tor1Δ with
pYX142-SU9-GFP [39].
Mitochondria
purification.
Mitochondria were isolated from yeast (from cultures
grown to OD600=1.0 in selective media) by differential
centrifugation followed by sucrose-gradient fractionation as described [40].
For 2D-DIGE, the purity of mitochondrial preparations was checked by western
blot analysis with anti-actin (Chemicon, 1:1000), anti-alkaline phosphatase
(Molecular Probe 1:1000), anti-Dol-P-Man synthase (Molecular Probes, 1:1000)
antibodies to control for contamination of cytoplasm, vacuolar membrane, and ER
membrane respectively. Only residual ER contamination was present in the
purified mitochondrial preparations (data not shown).
Mitochondrial
translation assay.
Unless otherwise stated, mitochondrial translation
assays were performed as described [25], except the following: all reactions
were carried out at 300C, gradient gels (6-20%, Figures 1 and 4;
15-22.5%, Figure 5) were used to resolve translation products and
electrophoresis was conducted at a constant current of 30 mA.
Chronological
Life Span Assay.
Chronological life span was assayed as described
previously [9,18] Unless otherwise stated, viability was determined by
staining with 0.4% trypan blue.
Measurement
of mtDNA Copy Number.
The mtDNA copy
number was determined using a quantitative real-time PCR procedure as described
previously [41,42].
Northern
Analysis.
Northern blots were performed as described previously
[24,42]. Briefly, 5 μg of total RNA extracted from yeast (cultured to an OD600=1)
was separated on a 1.5% agarose-formaldehyde gel and then transferred to a
nylon membrane by capillary action. Radiolabeled probes were synthesized by PCR
with 32P-dCTP an added to the membranes in rapid-hyb buffer (GE
Healthcare) and incubated overnight at 42 0C. The membrane was
washed at room temperature with increasing stringency before visualization by
auto-radiography as described in the references cited above.
Western
Blot Analysis.
Western blots of mitochondria and total cell extracts
(from cultures at (OD600=1) was performed as described previously
[18,25]. Proteins were separated on a 10% SDS-PAGE, transferred to a PVDF
membrane, and incubated with the indicated primary and HRP-conjugated
anti-mouse secondary (Molecular Probes) antibodies as described previously
[18, 25]. Anti-Cox4p (MitoSciences) antibody (not used previously) was diluted
1:1000 for incubation with the blocked membrane.
2D-DIGE.
Mitochondrial
extraction followed steps mentioned in the "mitochondrial extraction" section.
2D-DIGE was conducted by the W.M. Keck Facility of Yale University (http://keck.med.yale.edu/dige/).
Briefly, protein samples were prepared by TCA-precipitation of mitochondrial
extracts from DBY2006 and tor1Δ. The samples were further cleaned
with 2-D Clean-Up Kit (GE Healthcare) and labeled with CyDye DIGE fluors
(Amersham). 50 μg of the labeled samples were resolved on a 2D gel
(Ettan DIGE system from Amersham). A representative 2D gel and the distribution
of up-regulated and down-regulated proteins is shown in Supplementary Figure 3.
In this study, 26 proteins that were up-regulated by 2-fold or more were
selected for MALDI-MS/MS analysis. We were able to unambiguously identify 11 of
these based on multiple peptide matches.
Flow
Cytometry.
All analysis was performed on a Beckton-Dickenson
FACSCalibur. Analysis of yeast ROS using DHE was performed as described
previously [9]. For measurement of mitochondrial potential, cells from a
growing culture were pelleted by centrifugation and washed with
phosphate-buffered saline (PBS). DiOC6 (Molecular Probes) was
diluted to a final concentration of 200 nM in PBS and used to resuspend the
cells. The cell suspension was then incubated for 30 minutes at 30 0C,
washed twice with PBS, and analyzed by flow cytometry using the FL3 channel
without compensation. For measurement of mitochondrial mass, cultured GFP
expressing yeast cells were pelleted, washed once and then re-suspended in PBS,
and subject to flow cytometry analysis using the FL1 channel without
compensation.
Acknowledgments
This
work was supported by grant DAAD19-00-1-0560 from the Army Research Office
awarded to G.S.S. The authors wish to thank Marc Chatenay-Lapointe and Maria
Lebedeva for assistance with Northern analysis, other members of the Shadel Lab
for constructive suggestions, and Dr. Janet Shaw for providing the
mitochondrial-GFP expression plasmid used in the study.
Conflicts of Interest
The authors have no conflict of interests to declare.
References
-
1.
Tissenbaum
H
and Guarente
L.
Model organisms as a guide to mammalian aging.
Dev Cell.
2002;
2:
9
-19.
[PubMed]
.
-
2.
Mair
W
and Dillin
A.
Aging and survival: the genetics of life span extension by dietary restriction.
Annu Rev Biochem.
2008;
77:
727
-754.
[PubMed]
.
-
3.
Guarente
L
Mitochondria--a nexus for aging, calorie restriction, and sirtuins.
Cell.
2008;
132:
171
-176.
[PubMed]
.
-
4.
Wallace
D
A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine.
Annu Rev Genet.
2005;
39:
359
-407.
[PubMed]
.
-
5.
Costanzo
MC
and Fox
TD.
Control of mitochondrial gene expression in Saccharomyces cerevisiae.
Annu Rev Genet.
1990;
24:
91
-113.
[PubMed]
.
-
6.
Bonawitz
ND
, Clayton
DA
and Shadel
GS.
Initiation and beyond: multiple functions of the human mitochondrial transcription machinery.
Mol Cell.
2006;
24:
813
-825.
[PubMed]
.
-
7.
Balaban
RS
, Nemoto
S
and Finkel
T.
Mitochondria, oxidants, and aging.
Cell.
2005;
120:
483
-495.
[PubMed]
.
-
8.
Bonawitz
ND
and Shadel
GS.
Rethinking the mitochondrial theory of aging: the role of mitochondrial gene expression in lifespan determination.
Cell Cycle.
2007;
6:
1574
-1578.
[PubMed]
.
-
9.
Bonawitz
ND
, Rodeheffer
MS
and Shadel
GS.
Defective mitochondrial gene expression results in reactive oxygen species-mediated inhibition of respiration and reduction of yeast life span.
Mol Cell Biol.
2006;
26:
4818
-4829.
[PubMed]
.
-
10.
Hlavatá
L
, Nachin
L
, Jezek
P
and Nyström
T.
Elevated Ras/protein kinase A activity in Saccharomyces cerevisiae reduces proliferation rate and lifespan by two different reactive oxygen species-dependent routes.
Aging Cell.
2008;
7:
148
-157.
[PubMed]
.
-
11.
Barros
M
, Bandy
B
, Tahara
E
and Kowaltowski
A.
Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae.
J Biol Chem.
2004;
279:
49883
-49888.
[PubMed]
.
-
12.
Wullschleger
S
, Loewith
R
and Hall
MN.
TOR signaling in growth and metabolism.
Cell.
2006;
124:
471
-484.
[PubMed]
.
-
13.
Kunz
J
, Henriquez
R
, Schneider
U
, Deuter-Reinhard
M
, Movva
NR
and Hall
MN.
Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression.
Cell.
1993;
73:
585
-596.
[PubMed]
.
-
14.
Kaeberlein
M
, Powers
R 3rd
, Steffen
K
, Westman
EA
, Hu
D
, Dang
N
, Kerr
EO
, Kirkland
KT
, Fields
S
and Kennedy
BK.
Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients.
Science.
2005;
310:
1193
-1196.
[PubMed]
.
-
15.
Powers
R 3rd
, Kaeberlein
M
, Caldwell
SD
, Kennedy
BK
and Fields
S.
Extension of chronological life span in yeast by decreased TOR pathway signaling.
Genes Dev.
2006;
20:
174
-184.
[PubMed]
.
-
16.
Vellai
T
, Takacs-Vellai
K
, Zhang
Y
, Kovacs
AL
, Orosz
L
and Müller
F.
Genetics: influence of TOR kinase on lifespan in C. elegans.
Nature.
2003;
426:
620
[PubMed]
.
-
17.
Kapahi
P
, Zid
BM
, Harper
T
, Koslover
D
, Sapin
V
and Benzer
S.
Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway.
Curr Biol.
2004;
14:
885
-890.
[PubMed]
.
-
18.
Bonawitz
ND
, Chatenay-Lapointe
M
, Pan
Y
and Shadel
GS.
Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression.
Cell Metab.
2007;
5:
265
-277.
[PubMed]
.
-
19.
Schieke
SM
, Phillips
D
, McCoy
JP Jr
, Aponte
AM
, Shen
RF
, Balaban
RS
and Finkel
T.
The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity.
J Biol Chem.
2006;
281:
27643
-27652.
[PubMed]
.
-
20.
Urban
J
, Soulard
A
, Huber
A
, Lippman
S
, Mukhopadhyay
D
, Deloche
O
, Wanke
V
, Anrather
D
, Ammerer
G
, Riezman
H
, Broach
JR
, De
Virgilio C
, Hall
M
and Loewith
R.
Sch9 is a major target of TORC1 in Saccharomyces cerevisiae.
Mol Cell.
2007;
26:
663
-674.
[PubMed]
.
-
21.
Fabrizio
P
, Pozza
F
, Pletcher
SD
, Gendron
CM
and Longo
VD.
Regulation of longevity and stress resistance by Sch9 in yeast.
Science.
2001;
292:
288
-290.
[PubMed]
.
-
22.
Lavoie
H
and Whiteway
M.
Increased respiration in the sch9Δ mutant is required for increasing chronological life span but not replicative life span.
Eukaryot Cell.
2008;
7:
1127
-1135.
[PubMed]
.
-
23.
Bandhakavi
S
, Xie
H
, O'Callaghan
B
, Sakurai
H
, Kim
D
and Griffin
T.
Hsf1 activation inhibits rapamycin resistance and TOR signaling in yeast revealed by combined proteomic and genetic analysis.
PLoS ONE.
2008;
3:
e1598
[PubMed]
.
-
24.
Rodeheffer
MS
, Boone
BE
, Bryan
AC
and Shadel
GS.
Nam1p, a protein involved in RNA processing and translation, is coupled to transcription through an interaction with yeast mitochondrial RNA polymerase.
J Biol Chem.
2001;
276(11):
8616
-8622.
[PubMed]
.
-
25.
Rodeheffer
MS
and Shadel
GS.
Multiple interactions involving the amino-terminal domain of yeast mtRNA polymerase determine the efficiency of mitochondrial protein synthesis.
J Biol Chem.
2003;
278:
18695
-18701.
[PubMed]
.
-
26.
Bryan
AC
, Rodeheffer
MS
, Wearn
CM
and Shadel
GS.
Sls1p is a membrane-bound regulator of transcription-coupled processes involved in Saccharomyces cerevisiae mitochondrial gene expression.
Genetics.
2002;
160:
75
-82.
[PubMed]
.
-
27.
Ulery
TL
, Jang
SH
and Jaehning
JA.
Glucose repression of yeast mitochondrial transcription: kinetics of derepression and role of nuclear genes.
Mol Cell Biol.
1994;
14:
1160
-1170.
[PubMed]
.
-
28.
Amiott
EA
and Jaehning
JA.
Mitochondrial transcription is regulated via an ATP "sensing" mechanism that couples RNA abundance to respiration.
Mol Cell.
2006;
22:
329
-338.
[PubMed]
.
-
29.
Hardie
DG
, Carling
D
and Carlson
M.
The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell.
Annu Rev Biochem.
1998;
67:
821
-855.
[PubMed]
.
-
30.
Inoki
K
, Zhu
T
and Guan
KL.
TSC2 mediates cellular energy response to control cell growth and survival.
Cell.
2003;
115, 577
-590.
[PubMed]
.
-
31.
Beck
T
and Hall
M.
The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors.
Nature.
1999;
402:
689
-692.
[PubMed]
.
-
32.
Liu
Z
and Butow
R.
Mitochondrial retrograde signaling.
Annu Rev Genet.
2006;
40:
159
-185.
[PubMed]
.
-
33.
Carlson
M
Glucose Repression in yeast.
Curr Opin Microbiol.
1999;
2(2):
202
-207.
[PubMed]
.
-
34.
Dillin
A
, Hsu
AL
, Arantes-Oliveira
N
, Lehrer-Graiwer
J
, Hsin
H
, Fraser
AG
, Kamath
RS
, Ahringer
J
and Kenyon
C.
Rates of behavior and aging specified by mitochondrial function during development.
Science.
2002;
298:
2398
-2401.
[PubMed]
.
-
35.
Schulz
T
, Zarse
K
, Voigt
A
, Urban
N
, Birringer
M
and Ristow
M.
Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress.
Cell Metab.
2007;
6:
280
-293.
[PubMed]
.
-
36.
Piper
PW
, Harris
NL
and MacLean
M.
Preadaptation to efficient respiratory maintenance is essential both for maximal longevity and the retention of replicative potential in chronologically ageing yeast.
Mech Ageing Dev.
2006;
127:
733
-740.
[PubMed]
.
-
37.
Cassanova
N
, O'Brien
KM
, Stahl
BT
, McClure
T
and Poyton
R.
Yeast flavohemoglobin, a nitric oxide oxidoreductase, is located in both the cytosol and the mitochondrial matrix: effects of respiration, anoxia, and the mitochondrial genome on its intracellular level and distribution.
J Biol Chem.
2005;
280:
7645
-7653.
[PubMed]
.
-
38.
Burke
D
, Dawson
D
and Stearns
T.
Burke D, Dawson D, Stearns T.
Gene Replacement
Woodbury, USA
Cold Spring Harbor Laboratory Press
55
-59.
Methods in Yeast Genetics.
2000;
.
-
39.
Frederick
R
, McCaffery
J
, Cunningham
K
, Okamoto
K
and Shaw
J.
Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway.
J Cell Biol.
2004;
167:
87
-98.
[PubMed]
.
-
40.
Meisinger
C
, Pfanner
N
and Truscott
K.
Xiao W.
Isolation of Yeast Mitochodria
Totowa, USA
Humana Press
33
-39.
Methods in Molecular Biology, vol 313: Yeast Protocols.
2006;
.
-
41.
Taylor
SD
, Zhang
H
, Eaton
JS
, Rodeheffer
MS
, Lebedeva
MA
, O'rourke
TW
, Siede
W
and Shadel
GS.
The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae.
Mol Biol Cell.
2005;
16:
3010
-3018.
[PubMed]
.
-
42.
Lebedeva
MA
and Shadel
GS.
Cell cycle- and ribonucleotide reductase-driven changes in mtDNA copy number influence mtDNA Inheritance without compromising mitochondrial gene expression.
Cell Cycle.
2007;
6:
2048
-2057.
[PubMed]
.