Introduction
More
than eighty years ago, Alexis Carrel demonstrated that leukocyte extracts, like
embryonic tissue extracts, stimulate multiplication of fibroblasts in vitro,
and suggested that leukocytes can bring growth-activating substances to
tissue-specific cells [1]. More recently,
lymphocytes and also monocyte derived cells (MDC) were shown to promote tissue
growth and regeneration [2-7].
It
has been suggested that only one of the many functions of lymphocytes is their
participation in host immune responses since lymphoid cells as "trephocytes"
also participate in a number of physiological processes aimed at maintaining
homeostasis [2]. In addition, abundance of
tumor-associated macrophages is correlated with poor prognosis. It has been
hypothesized that besides normal trophic functions, the MDC promote tumor
progression and metastasis [8]. However, while a
lot of work has been done to examine the influence of various growth factors
and cytokines produced by lymphocytes and MDC on the cell cycle and death in
vitro [9-14], little is known about
interactions between these immune system-related mesenchymal cells and
tissue-specific cells in vivo.
The
biological role of intraepithelial immune system-related cells has remained an
enigma to researchers for many years. It is still widely believed that the only
role of MDC and gamma delta T cells in epithelial tissues such as the skin,
gut, and lung is in maintaining tissue integrity, defending against pathogens,
regulating inflammation, wound healing, and monitoring neighboring cells for
signs of damage or disease [5,6,15,16].
However, there are tissues which do not communicate with the outer environment,
such as the ovarian corpus luteum (CL), in which MDC and T cells accompany
differentiation and demise of epithelial cells [3,17,18]. Therefore, we hypothesize that the primary role of intraepithelial
MDC and T cells is to maintain tissue homeostasis, such as proliferation,
differentiation, and preservation of epithelial or parenchymal cells in a
functional state (immune physiology).
Alteration of immune physiology can by
itself cause alteration of tissue function (immune pathology), such as rheumatic
[19] and degenerative diseases [20]. Secondarily, if needed, the MDC and T cells are
converted into effectors of immunity defending against pathogens (immune
surveillance). Moreover, the role of immune system components in the regulation
of tissue physiology and pathology should be viewed in context with resident
mesenchymal cells, such as vascular pericytes derived from stromal fibroblasts,
as well as neuronal signals.
This review article focuses on the role of immune
system-related cells and molecules they produce in the regulation of epithelial
and parenchymal cell proliferation, differentiation, and aging, and describes a
theory of the so called Tissue Control System (see below) in the reproductive
tract, which may be universal for other tissues as well. Some implications of
immune physiology for augmentation of cancer and efficient utilization of
regenerative medicine are also suggested.
THE TISSUE CONTROL SYSTEM THEORY
To
study the role of immune physiology in homeostasis of tissues in general, the
tissues with fast cellular development and demise are essential. The female
reproductive tract tissues represent one of the most dynamic and active
structures within the mammalian body. Our studies in the late 1970s [21-24] and early 1980s [25,26]
resulted in the concept of a wider role of the immune system (immune system
cells and vascular pericytes), the so called Tissue Control System (TCS), in
regulation of ovarian function [27]. The TCS
theory was further refined when the role of autonomic innervation in the
regulation of quantitative aspects in tissues, including ovarian follicular
selection, was added, [28,29] and the TCS theory
was revised [30,31]. Autonomic innervation plays
an important role in determination of the extent of tissue development since an
elimination of limited areas of the cephalic neural crest in stage 9 or 10
chick embryos markedly reduced the size of the thymus gland or resulted in its
absence. Small thymic lobes contained both normal thymocytes and epithelial
cells, but showed delayed development [32]. More
recently, a role for the immune system-related cells in the regulation of
ovarian aging [33,34] and regulation of
asymmetric cell division of germ cell progenitors, giving rise to new germ
cells during the fetal period and adulthood [35-37],
have been described.
Basic "tissue
control unit" and "immune physiology"
The
TCS consists of immune system-related cells (MDC and T and B lymphocytes),
vascular pericytes, and autonomic innervation. While immune reactions are
directed against foreign substances (immune surveillance), the TCS is proposed
to regulate regeneration, preservation and aging of tissue specific cells
("immune physiology") including the female reproductive tissues [27,30,33]. Ovary, uterus and, in the case of
pregnancy, the placenta exhibit periodic growth and regression, which are
extremely rapid and are accompanied by changes in rates of blood flow.
Therefore, it is not surprising that angiogenesis and remodeling of the local
epithelium and vascular bed occur as a normal process in these tissues [38-41].
The
basic "tissue control unit" (TCU) is associated with tissue
microvasculature. Monocyte-derived cells (marked M in Figure 1) interact with
vascular pericytes (P), and both cell types regulate, via growth factors and
cytokines, proliferation, differentiation, and apoptosis of tissue specific
epithelial (Ep) and endothelial cells (En). The influence of TCU on endothelial
cells plays an important role in the control of homing of tissue-committed
circulating MDC and T cells, a process which is mediated by highly regulated
vascular adhesion molecules and by chemoattractant factors. The intraepithelial
MDC [dendritic cell precursors (DCP) and dendritic cells (DC)], T cells (T),
and natural autoantibodies (three types of IgM: IgM1, IgM2 and IgM3 - see
later, and one type of IgG) [4] play an important
role in the control of qualitative aspects (differentiation and aging)
of tissue cells, and autonomic innervation controls quantitative aspects
of tissues by regulation of TCU activity [AI (+ or -)] [3,4].
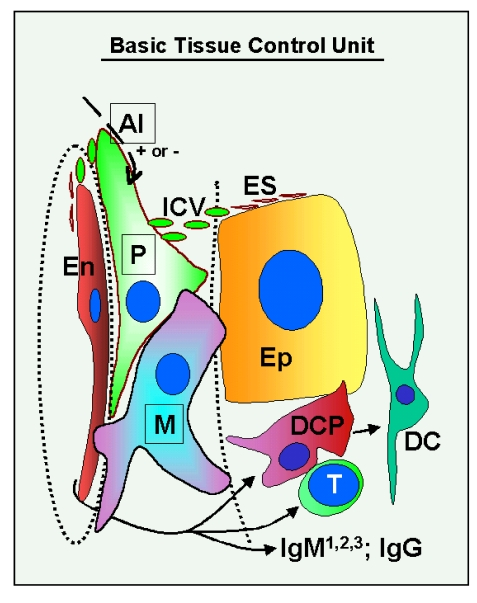
Figure 1. Schematic drawing of the basic "tissue control unit," which consists of monocyte-derived cells (marked M in the figure), vascular pericytes (P), and autonomic innervation (AI, dashed arrow), and the involvement of other components of the tissue control system (solid arrows). Monocyte-derived
cells physically interact with adjacent epithelial (Ep) and
endothelial cells (En) through the basement membranes (dotted lines),
and influence pericytes, which secrete intercellular vesicles
(ICV). These vesicles collapse into the so-called empty spikes (ES)
releasing their content (growth factor/cytokine) after reaching target
cells. The activity of pericytes is stimulated or inhibited by
autonomic innervation (+ or -) which controls quantitative aspects
of tissues. Interaction of MDC with endothelial cells may stimulate
homing of T lymphocytes (T) and monocyte-derived dendritic cell precursors
(DCP; also known as veiled cells) differentiating into mature dendritic
cells (DC). The dendritic cell precursors and T cells interact themselves
and stimulate advanced differrentiation of epithelial cells.
IgMs regulate early (IgM1), mid (IgM2), and late differentiation
(apoptosis) of epithelial cells (IgM3), and IgG associates with aged cells
(see Figure 2 and 3). The monocyte-derived cell system (including
intraepithelial DCP and mature DC) is postulated to play a dominant role
in the regulation of qualitative aspects of tissue-specific cells, including
expression of ligands for intraepithelial T cells and regulating autoantibody action.
Monocyte-derived cells also carry "stop effect" information (Figure 10B),
presumptively encoded at the termination of immune adaptation (Figure 10A),
which determines the highest state of epithelial cell differentiation allowed
for a particular tissue. For details see Ref. [3,4,33]. Reprinted from Ref. [4], © Antonin Bukovsky.
Complete
TCS pathway reflects immune system phylogeny
Examples
of complete TCS involvement in the regulation of cellular differentiation, from
the stem to mature and aged cells, can be found in some stratified epithelial
tissues, such as uterine ectocervix. The stratified epithelium of uterine
ectocervix consists of four layers of epithelial cells, basal (b), parabasal
(pb), intermediate (im), and superficial cells (s; see Figure 2A). The basal
layer is formed by a single row of basal or stem cells. The parabasal layer
contains several layers of parabasal (young) epithelial cells, the intermediate
layer consists of multiple layers of mature epithelial cells, and the
superficial layer is formed by several layers of aged cells. These four
morphologically distinct layers are divided by three interfaces - b/pb, pb/im,
and im/s interface. The parabasal and intermediate layers can be divided into
the lower, mid, and upper layers, the superficial layer into the lower and
upper layers.
Mesenchymal
cells are present in the lamina propria and invade among epithelial cells.
Staining for CD14 of primitive MDC (Figure 2A) shows small MDC in the lamina
propria but not within the epithelium. Figure 2B, shows association of CD14
cells with the basement membrane (arrows) and extension among basal epithelial
cells (arrowhead). This indicates that primitive MDC may stimulate
proliferation of stem cells. The CD14 is a lipopolysaccharide receptor, and is
involved in the stimulation of cell proliferation [42].
Similar association of CD14 primitive MDC with proliferating epithelial cells
was detected in ovarian cancers (see later). Staining for Ki67 (inset, Figure 2A)
shows that this marker of proliferation is expressed in the nuclei of parabasal
cells adjacent to the b/pb interface. This indicates that in the stratified
epithelium of ectocervix, Ki67 is expressed in postmitotic cells leaving the
basal layer and beginning differentiation.
These
observations indicate that primitive MDC accompany proliferation of basal
epithelial (stem) cells.
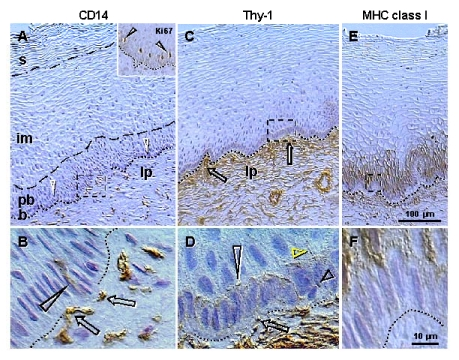
Figure 2. Peroxidase immunohistochemistry (brown color) of stratified epithelium of uterine ectocervix as indicated above columns and in the inset.
(A) CD14
primitive MDC in lamina propria (lp) associate with the epithelium basement
membrane (dotted line). Dashed box indicates detail shown in (B). b,
basal layer; pb, parabasal layer; im, intermediate layer; s,
superficial layer. Arrowheads, basal/parabasal interface; dashed line,
parabasal/intermediate interface; dashed/dotted line,
intermediate/superficial interface. Ki67 staining (inset) of epithelial
cells in lower parabasal layer (arrowheads). (B) CD14 MDC (arrows)
exhibit extensions among basal cells (arrowhead). (C) Pericytes of
microvasculature (arrows) associate with the basement membrane. (D)
Detail from (C) shows intercellular Thy-1 vesicles (arrow) secreted by
pericytes and migrating among basal cells (short black arrowhead) to
basal/parabasal interface (long
arrowhead). Yellow arrowhead indicates residual empty structures
("spikes"). (E) Strong MHC class I expression (W6/32
antibody specific for heavy chain) is characteristic of para-basal cells,
and diminishes in lower intermediate layers. Dashed box indicates detail
shown in (F). (F) Basal cells show no MHC class I expression.
Reprinted from Ref. [4], © Antonin Bukovsky.
Recognition
at the cell surface
Most of the molecules involved in the TCS
pathway belong to the immunoglobulin (Ig) superfamily of molecules. It has been
suggested that the involvement of Ig-related molecules in tissue interactions
is more primitive than their involvement in the immune system and the immune
functions evolved from the sets of molecules mediating tissue interactions [43]. One of them, the Thy-1 differentiation protein,
consists of a single Ig domain and represents the most primitive and ancestral
member of the Ig-superfamily. The Ig-related molecules have a diversity of
functions, but in most cases the common denominator is recognition at the cell
surface [44]. Also, the only function of Thy-1
differentiation protein and other Ig-related molecules is to mediate
recognition, with the consequences of recognition being due to the
differentiated state of the cells. It requires that the correct ligand and
receptor are expressed on the appropriate cells at the right time [43].
Staining
for Thy-1 differentiation protein (Figure 2C) shows pericytes associated with
microvasculature (arrows) adjacent to the basement membrane. Detail of Thy-1
staining (Figure 2D) shows that pericytes secrete intercellular vesicles, which
migrate among basal epithelial cells to the b/pb interface, where they collapse
into empty structures ("spikes"). Hence, targets for Thy-1 vesicles
appear to be parabasal cells adjacent to the b/pb interface, i.e., epithelial
cells expressing Ki67 and entering differentiation.
These
intercellular Thy-1 vesicles have been shown by immunoelectron microscopy to
exhibit Thy-1 surface expression and to contain a substance lacking Thy-1
staining [30]. They may represent a unique
paracrine mechanism, so called "targeted delivery," by which certain
growth factors (vesicle contents) are delivered to certain type/stage specific
target cells expressing receptor for Thy-1 ligand. However, the receptor for
Thy-1 has not yet been identified. One possibility is that the Ki67+ cells
entering differentiation are the targets for Thy-1+ intercellular vesicles.
Also, there is a lack of expression of major histocompatibility complex (MHC)
class I molecules in epithelial cells adjacent to the basement membrane, but
strong staining in parabasal cells (Figure 2E and F). Hence, MHC
class I molecules could be involved in the recognition of the Thy-1 ligand.
Targeted
delivery of some tissue non-specific (stimulating many types of tissues) growth
factors to particular tissue cells by intercellular Thy-1 vesicles could be
enabled by tissue specificity of Thy-1 glycoprotein carbohydrate moieties [45].
MHC
class I and class II molecules are other Ig superfamily members. Figure 3A
shows that large quantities of HLA-DR molecules are secreted by precursors of
dendritic cells among epithelial cells in the mid parabasal layer (arrows).
This site-specific HLA-DR secretion is particularly evident when DC precursors
are compared with inactive MDC in the lamina propria (lp) or mature DC in
intermediate epithelial layers (arrowheads).
These
observations indicate that recognition at the cell surface by Ig superfamily
members may play an important role in immune physiology.
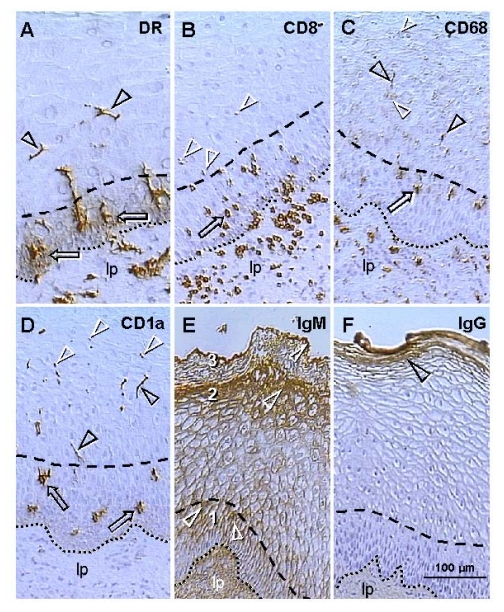
Figure 3. Uterine ectocervix immunohistochemistry as indicated above columns. (A) Dendritic cell
(DC) precursors secrete HLA-DR among parabasal cells (arrows) and
differentiate into mature DC (arrowheads). (B) T cells migrate
through parabasal layer (arrow) to parabasal/intermediate interface (dashed
line) and show fragmentation after entering the intermediate layer
(arrowheads). (C) Transformation of DC precursors into mature DC at
the top of parabasal layer is associated with CD68 expression (arrow).
Mature DC (black arrowheads) secrete CD68 material in intermediate layer
accompanying mature (intermediate) and aged (superficial) epithelial cells
(white arrowheads). (D) CD1a is expressed by DC precursors (arrows)
and mature DC (black arrowheads). Mature DC (Langerhans' cells) undergo
fragmentation in the mid intermediate layer (white arrowheads). (E)
Strong IgM binding (arrowheads) in upper parabasal [1], upper intermediate
[2] and upper superficial layers [3]. (F) IgG binds to the entire
superficial layer. For abbreviations see Figure 2. Reprinted from Ref. [4], © Antonin Bukovsky.
Degeneration
of intraepithelial T cells and MDC and differentiation of epithelial cells
T
cells expressing CD8, which is another member of the Ig superfamily, accumulate
in the lamina propria, enter the epithelium, and migrate through the parabasal
layers (arrow, Figure 3B), toward the pb/im interface (dashed line). T cells
entering lower intermediate layers exhibit fragmentation (arrowheads). No T
cells were detected in the mid intermediate layers or at the epithelial
surface.
The
CD68 epitope of mature intraepithelial MDC, a mucin-like molecule belonging to
the lysosomal-associated membrane protein family [46],
is expressed by MDC in the lamina propria (Figure 3C). However, within
epithelium, CD68 appears during transformation of DC precursors into mature DC,
in the upper parabasal layers (arrow). Mature DC (black arrowheads) secrete
CD68 among intermediate epithelial cells, and CD68 mucin-like molecules
accompany advanced differentiation of epithelial cells (white arrowheads)
including aging in surface layers.
Staining
for CD1a, an Ig-related molecule characteristic for intraepithelial Langerhans'
cells, was not detected in the lamina propria (Figure 3D). Dendritic cell
precursors (arrows) and mature DC (black arrowheads) were stained. Mature DC
reaching mid intermediate layers exhibited fragmentation (white arrowheads)
similar to that of T cells in the lower intermediate layers.
It
is apparent that intraepithelial T cells crossing the pb/im interface
degenerate (Figure 3B), and such apoptosis may be required for a release of
substances enabling maturation of epithelial cells. In addition, Figure 2A, 3A,
and 3C and D, show that intraepithelial MDC also exhibit morphological and immunohistochemical
features accompanying their maturation and demise.
These
observations suggest that degeneration of intraepithelial T cells and MDC may
be required for advanced differentiation of epithelial cells.
Association
of natural IgMs and IgG with epithelial differentiation
Natural
autoantibodies are present in the blood of normal healthy individuals, and they
are almost exclusively IgM antibodies, although some IgG and IgA natural
autoantibodies can also be detected, that bind to a variety of self-antigens,
including self IgG [47,48]. When compared to
IgG, the IgM molecules appear earlier in phylogeny and ontogeny [49,50].
Staining
of ectocervical epithelium for IgM is shown in Figure 3E. Basal and lower
parabasal layers are unstained, but IgM binding increases toward the pb/im
interface (#1). In the intermediate layers, a similar increase of IgM binding
is apparent toward the im/s interface (#2). In the superficial layers the most
prominent staining is evident at the epithelial surface (#3). Hence, there is
high IgM binding to the upper cells in the parabasal, intermediate, and surface
layers (white arrowheads). IgG does not bind to the basal, parabasal or
intermediate cells, but shows binding to the entire superficial layer (arrowhead,
Figure 3F).
These
data indicate that natural autoantibodies exhibit a stage-specific
(differentiation-dependent) binding to epithelial cells. Similar stage-specific
binding to epidermis was also detected for natural IgM and IgG autoantibodies
in normal human sera [51].
Interaction
of intraepithelial T cells and MDC and T cell demise
Basal
and parabasal layers of normal ectocervical epithelium show the presence of T
cells and MDC. A possibility exists that, beside interaction with epithelial
cells, these mesenchymal cell types may interact with each other. Figure 4
shows the pb/im interface in detail, with staining for HLA-DR MDC (A), CD8 for
T cells (B) and both (C). T cells appear to assist differentiation of DC (white
arrowheads) and exhibit an unusual elongated shape accompanied by HLA-DR
expression (white arrows). Above this interface, the mature DC (yellow
arrowhead) accompany fragmentation of T cells (yellow arrows).
These
data suggest that transition of parabasal into the intermediate epithelial
cells at the pb/im interface is associated with transformation of DC precursors
into mature DC with the assistance of activated (HLA-DR+) T cells. The T cells
entering intermediate layers show a loss of HLA-DR expression and undergo
fragmentation and demise with the assistance of mature DC.
![Uterine cervix dual color
immunohistochemistry (HLA-DR peroxidase/CD8 FITC) viewed in dark field
visible light (A), incident fluorescence (B) and dark field
fluorescence (C). (A) Interface (dashed line) between
parabasal and intermediate layers. White arrowhead shows differentiating
DC, yellow arrowhead shows mature DC. Arrow indicates activated T cell with
HLA-DR expression (see below). (B) White arrow indicates T cell
exhibiting unusual elongated shape at the interface. Yellow arrows indicate
residual CD8 expression in fragmented T cell among adjacent im epithelial
cells. (C) Activated T cell with HLA-DR expression (white arrow)
interacts with differentiating DC (white arrowhead). Mature DC (yellow
arrowhead) accompany T cell fragmentation (yellow arrows). Reprinted from
Ref. [4], © Antonin Bukovsky.](/article/100024/figure/F4/large)
Figure 4.
Uterine cervix dual color
immunohistochemistry (HLA-DR peroxidase/CD8 FITC) viewed in dark field
visible light (A), incident fluorescence (B) and dark field
fluorescence (C). (A) Interface (dashed line) between
parabasal and intermediate layers. White arrowhead shows differentiating
DC, yellow arrowhead shows mature DC. Arrow indicates activated T cell with
HLA-DR expression (see below). (B) White arrow indicates T cell
exhibiting unusual elongated shape at the interface. Yellow arrows indicate
residual CD8 expression in fragmented T cell among adjacent im epithelial
cells. (C) Activated T cell with HLA-DR expression (white arrow)
interacts with differentiating DC (white arrowhead). Mature DC (yellow
arrowhead) accompany T cell fragmentation (yellow arrows). Reprinted from
Ref. [4], © Antonin Bukovsky.
LESSONS FROM MAMMALIAN FEMALE REPRODUCTION
Mammalian female reproduction is much
more complex when compared to males and non-mammalian females. Gonads of adult
males contain germ cells (spermatogonia), which produce fresh gametes. Such
germ cells are, however, not present in adult female gonads of higher
vertebrates, including mammals. Because of that, a dogma evolved about fifty
years ago that the process of oogenesis in the animal kingdom follows a uniform
pattern, of which there are two main variants.
One variant is that the oogenesis appears to continue either uninterruptedly or
cyclically throughout reproductive life - e.g. most teleosts, all amphibians,
most reptiles and relatively few mammals. The other variant is that the
oogenesis occurs only in fetal gonads, and oogonia neither persist nor divide
mitotically during sexual maturity - e.g. cyclostomes, elasmobranchs, a few
teleosts, perhaps some reptiles, all birds, monotremes, and with a few
possible exceptions, all eutherian mammals [52,53].
Nevertheless, in the early 1970s, this belief was felt unwarranted due to a
lack of detailed study of adult mammalian ovaries. A thorough reexamination of
oogenesis, using modern techniques at well-defined stages of the reproductive
cycle, was suggested [54].
In
addition, it is also currently believed that oogonia in fetal ovaries of higher
vertebrates originate from primordial germ cells, which differentiate into oogonia
producing definitive oocytes. However, it is apparent that germ cells are
present at the ovarian surface of midpregnancy human fetuses [55]. Our observations support the view that primordial
germ cells play a role in the commitment of the surface ovarian stem cells
(OSC) toward production of secondary germ cells, and then degenerate (reviewed
in [56]). Secondary germ cells are formed in
fetal and adult human ovaries by asymmetric division of OSC, with the
assistance of MDC and T cells [35,36,57].
Mammalian
ovarian compartments belong to those structures showing most pronounced
morphological (cellular proliferation, differentiation and regression) and
functional changes within the body. Regulation of ovarian function is quite
complex, involving interactions between follicular compartments (oocyte,
granulosa, and theca cells), as well as the influence of sex steroids produced
by follicles, CL and interstitial glands originating from the theca of
degenerating follicles. Additionally, communication of the ovary with the
hypothalamo-pituitary system and the influence of gonadotropins, autonomic
innervation, growth factors and cytokines produced by mesenchymal cells derived
from the immune system, all regulate functions of ovarian compartments. While
gonadotropins are essential for follicular maturation and ovulation [58], autonomic innervation is necessary for the
regulation of follicular selection [59,60].
Interactions
between the immune system and ovary are numerous, as immune cells are
associated with regulation at every level of the hypothalamo-pituitary-ovarian
axis, regulating growth and regression of both follicles and CL [61-63].
Oogenesis
in fetal and adult human ovaries
Earlier observations indicated that
secondary germ cells develop in fetal ovaries from the somatic OSC, i.e.
"germinal" (surface) epithelium of the ovary [64].
However, until the work of Dustin [65], there
were no questions regarding the fate of primordial germ cells within developing
ovaries. He recognized two kinds of cells in the germ-line history of
amphibians: [1] primordial germ cells, which populated the developing gonad,
differentiated into gonocytes, and degenerated, and [2] secondary germ cells
originating from the ovarian "germinal" epithelium, which differentiated
into definitive oocytes.
Rubaschkin
[66] suggested division of the history of the
germ cell route (Keimbahn) into three periods. The first period begins with the
differentiation of primordial germ cells, which, however, do not have a
perspective to become definitive gametes (Urgeschlechtszellen). The gonadal
development is associated with the establishment of the so called germinal
epithelium (Keimepithel). The second period is associated with the appearance
of female or male sex specific cells (Ureier or Ursamenzellen). The third
period deals with the development of the sex-specific glands.
The
germ cell route of Rubaschkin again raised a question of the fate of primordial
germ cells [67]. Winiwarter and Sainmont [68] suggested that these cells degenerate after
reaching the sex gland, and that definitive germ cells arise from the ovarian
"germinal" epithelium.
Our
observations indicate that secondary germ cell development in midpregnant human
fetal ovary is triggered by MDC and T cells (Figure 5). The germ cells are
depleted of major histocompatibility complex class I (MHC-I) expression (red
asterisks, Figure 5A and B), and they originate by asymmetric division (white
arrowheads) from OSC densely expressing MHC-I (yellow asterisks). A symmetric
division of the germ cells, required for crossing over of chromosomes [69], follows (yellow arrowhead, panel B). The germ
cells (gc) take on an ameboid shape (dashed line, no hematoxylin counterstain),
and enter the adjacent ovarian cortex. Primitive CD14-expressing MDC interact
with OSC (arrowhead, panel C), and accompany (arrowhead, panel D) subsequent
symmetric division of the germ cells (asterisks). T cells expressing CD8 (panel
E) and showing activation (HLA-DR expression, panel F) accompany (black
arrowheads) asymmetric division of OSC (white arrowheads, panels E and F). Note
that during asymmetric division the emerging germ cells daughters (red
asterisks) are substantially larger than OSC daughter cells (yellow asterisks).
The activated (HLA-DR+) MDC are associated with growing (gf and arrowheads,
panel G) but not resting primordial follicles (pf) [36,70].
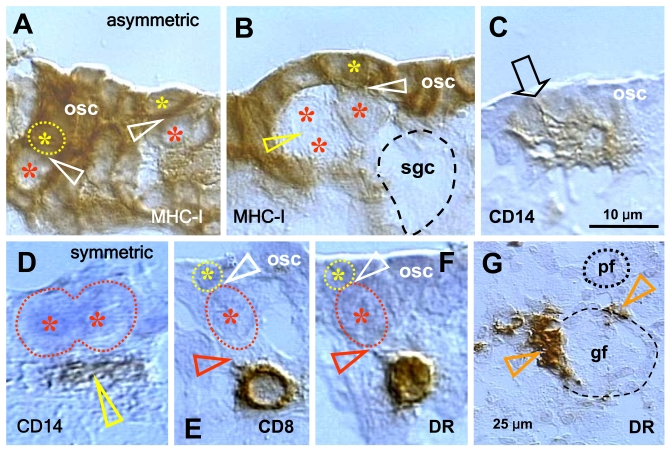
Figure 5. Expression of MHC class I heavy chain (MHC-I), CD14 of primitive MDC, CD8 of T cells, and HLA-DR (DR) of activated MDC and T cells, as indicated in the panels, in human fetal ovary obtained at midpregnancy (24 weeks). Asymmetric division (white
arrowheads, panels A and B) of OSC (osc) gives rise to the
OSC (yellow asterisks) and the germ cell daughters (red asterisks).
Symmetric division of germ cells follows (yellow arrowhead, panel B),
which is required for crossing over, and the secondary germ cells (sgc)
attain the ameboid shape (dashed line, no hematoxylin counterstain) to
leave the OSC layer and enter cortex. CD14+ primitive MDC interact with the
OSC (arrow, panel C) and accompany (arrowhead, panel D)
symmetric division of secondary germ cells. CD8 T cells (panel E)
and DR+ cells of lymphocyte type (panel F) accompany (red
arrowheads) asymmetric division of OSC (white arrowheads) resulting in
emergence of secondary germ cells. DR+ MDC (arrowheads, panel G)
associate with growing (gf) but not resting primordial follicles (pf). Bar
in C for A-F. Adapted from Ref. [36],
© Humana Press.
Similar
emergence of secondary germ cells by asymmetric division of OSC triggered by
MDC and T cells has been observed in adult human ovaries [35,57] and expression of a meiosis marker, the
meiotic entry synaptonemal complex protein-3 (SCP3), in segments of tunica
albuginea and OSC, and in some oocytes of primordial follicles was detected in
functional adult human and monkey ovaries [71].
In addition, we also have shown emergence of secondary germ cells by asymmetric
division of OSC triggered by MDC and T cells in adult rat ovaries [72]. This indicates that MDC and T cells induce
emergence of secondary germ cells from the OSC in various mammalian species.
Adult human ovaries exhibiting
neo-oogenesis showed association of CD14 primitive MDC with OSC (arrows, Figure 6A). During asymmetric division, both the emerging germ cell daughter (red
asterisk) and the OSC daughter
cells (yellow asterisk) were accompanied by extensions of CD14 MDC (see color
matching arrowheads). It is apparent, however, that interaction of CD8 T cells
is unique for the emerging germ cells (red arrowheads, Figure 6B), see also Figure 5E and F. This suggests that the number of interacting CD8 T cells may
determine the number of emerging secondary germ cells in fetal and adult human
ovaries. As in fetal ovaries, asymmetric division resulted in the emerging germ
cell daughter being larger than the OSC daughter. The HLA-DR (activated) MDC
accompanied (arrowhead, Figure 6C) migration of germ cells exhibiting ameboid
shape through the dense upper ovarian cortex toward ovarian vessels, which they
entered [57].
![Origin of new oocytes
(neo-oogenesis), primordial follicles, and SCP3 expression in adult human
and monkey ovaries (A-M), and oogenesis in adult rat ovaries (N-P).
(A) During asymmetric division (white arrowhead), the CD14 MDC
interact with both the OSC daughter (yellow arrowhead) and germ cell
daughter (red arrowhead). (B) T lymphocytes, however, interact with
the germ cell daughter only (red arrowheads). (C) Ameboid germ cells
(dotted line) migrating through the dense ovarian cortex (oc) are
accompanied by activated MDC (arrowhead). (D) Asymmetrically
dividing OSC produce a new PS1+ germ cell (red asterisk) and CK+ progenitor
cell (yellow asterisk). (E) In the tunica albuginea (ta) germ cells
(asterisks) symmetrically divide (arrowhead). (F) Capture of oocyte
(o) from the blood circulation by an arm (a) of granulosa cell nest (n)
lining the venule lumen (vl); e, endothelial cells. (G) Oocyte nest
assembly. (H) Segments of tunica albuginea (ta) in ovaries with
follicular renewal (early luteal phase) showed strong SCP3 expression of
mesenchymal (arrowheads) OSC precursors under ovarian surface (os). (I)
Staining of OSC (osc and arrowhead) was apparent in other segments - note
lack of staining of tunica albuginea under developed OSC. (J)
Postovulatory human ovaries showed staining of oocyte nucleoli (arrowhead)
in some primordial follicles. (K) In monkey ovaries, similar
staining of oocyte nucleoli in some primordial follicles was observed (red
vs. white arrowhead). (L) Staining of paired chromosomes oocyte was
observed in human ovaries (inset shows higher magnification). (M)
Adult rat testis (positive control) showed staining of condensed chromosomes
in spermatogonia (red arrowhead) and progression of meiotic division in
primary spermatocytes (black arrowhead). Oogenesis in adult rat ovaries is
initiated by asymmetric division of OSC (white arrowhead, N) showing
unstained OSC daughter (yellow asterisk) and ZP+ (magenta color) germ cell
daughter (red asterisk) accompanied like in human ovaries by a lymphocyte
(black asterisk and brown color). Symmetric division of ZP+ oogonia
(asterisks, O) follows, and is accompanied (P) by MDC (yellow
arrowhead). Blue arrowheads in (P) indicate association of primitive
granulosa cells with this process. ZP, zona pellucida; LCA, leukocyte
common antigen; W6/25, marker of rat MDC. Details in text. Adapted A-C from Ref. [57], © Blackwell Munksgaard, D-G from Ref. [35], © Antonin Bukovsky, H-M from Ref. [71], © Landes Bioscience, N-P from Ref. [72], © Landes Bioscience.](/article/100024/figure/F6/large)
Figure 6.
Origin of new oocytes
(neo-oogenesis), primordial follicles, and SCP3 expression in adult human
and monkey ovaries (A-M), and oogenesis in adult rat ovaries (N-P).
(A) During asymmetric division (white arrowhead), the CD14 MDC
interact with both the OSC daughter (yellow arrowhead) and germ cell
daughter (red arrowhead). (B) T lymphocytes, however, interact with
the germ cell daughter only (red arrowheads). (C) Ameboid germ cells
(dotted line) migrating through the dense ovarian cortex (oc) are
accompanied by activated MDC (arrowhead). (D) Asymmetrically
dividing OSC produce a new PS1+ germ cell (red asterisk) and CK+ progenitor
cell (yellow asterisk). (E) In the tunica albuginea (ta) germ cells
(asterisks) symmetrically divide (arrowhead). (F) Capture of oocyte
(o) from the blood circulation by an arm (a) of granulosa cell nest (n)
lining the venule lumen (vl); e, endothelial cells. (G) Oocyte nest
assembly. (H) Segments of tunica albuginea (ta) in ovaries with
follicular renewal (early luteal phase) showed strong SCP3 expression of
mesenchymal (arrowheads) OSC precursors under ovarian surface (os). (I)
Staining of OSC (osc and arrowhead) was apparent in other segments - note
lack of staining of tunica albuginea under developed OSC. (J)
Postovulatory human ovaries showed staining of oocyte nucleoli (arrowhead)
in some primordial follicles. (K) In monkey ovaries, similar
staining of oocyte nucleoli in some primordial follicles was observed (red
vs. white arrowhead). (L) Staining of paired chromosomes oocyte was
observed in human ovaries (inset shows higher magnification). (M)
Adult rat testis (positive control) showed staining of condensed chromosomes
in spermatogonia (red arrowhead) and progression of meiotic division in
primary spermatocytes (black arrowhead). Oogenesis in adult rat ovaries is
initiated by asymmetric division of OSC (white arrowhead, N) showing
unstained OSC daughter (yellow asterisk) and ZP+ (magenta color) germ cell
daughter (red asterisk) accompanied like in human ovaries by a lymphocyte
(black asterisk and brown color). Symmetric division of ZP+ oogonia
(asterisks, O) follows, and is accompanied (P) by MDC (yellow
arrowhead). Blue arrowheads in (P) indicate association of primitive
granulosa cells with this process. ZP, zona pellucida; LCA, leukocyte
common antigen; W6/25, marker of rat MDC. Details in text. Adapted A-C from Ref. [57], © Blackwell Munksgaard, D-G from Ref. [35], © Antonin Bukovsky, H-M from Ref. [71], © Landes Bioscience, N-P from Ref. [72], © Landes Bioscience.
Dual
color immunohistochemistry has shown that during asymmetric division OSC
daughters retain cytokeratin expression (blue color, Figure 6D), but the
emerging secondary germ cell loses it and attains expression of meiotically
expressed PS1 carbohydrate (brown color) [73,74].
As in fetal ovaries, symmetric division of secondary germ cells follows (Figure 6E). The secondary germ cells entering ovarian venules enlarge to the size of
small oocytes and are caught in the deep ovarian cortex by the arms (a, Figure 6F)
of primitive granulosa cell nests (n) lining the venule lumen (vl). More
advanced nest-oocyte assembly resembling an occupied bird's nest is shown in Figure 6G. See [35] for more data.
These
observations indicate that secondary germ cells develop from OSC in adult human
ovaries and form new primordial follicles by assembly with granulosa cell
nests.
Expression
of meiotic entry SCP3 protein in adult human and monkey ovaries
In a recent study, Liu and colleagues compared expression
of meiotic marker SCP3 in fetal and functionally undefined adult human ovaries
[75]. The authors argued that SCP3 protein was
not detectable in the tunica albuginea, OSC or in oocytes of primordial
follicles in adult ovaries, and hence concluded that no meiotic oocytes are
present in ovaries during adulthood. In a subsequent commentary, Tilly and
Johnson [76] indicated that the lack of evidence
on neo-oogenesis in adult human females is not evidence of its absence, and on
the contrary that some data of Liu et al. [75]
support the existence of neo-oogenesis in adult women. Subsequently we reported
that using the same SCP3 antibody, immune-reactivity with segments of tunica
albuginea and OSC, and in some oocytes of primordial follicles in functional
adult human and monkey ovaries was detected [71].
Meiotic entry SCP3 protein is expressed
in precursor cells of OSC in some segments of tunica albuginea in functional
adult human ovaries (arrowhead, Figure 6H) and also in OSC cells of human
(panel I) and monkey ovaries [71]. Moreover, SCP3
immunostaining was observed in the nucleoli of oocytes in some primordial
follicles in adult human (arrowhead, panel J) and monkey
ovaries (red vs. white arrowhead, panel K). Earlier, Tres had reported that
male germ cells exhibit nucleolar SCP3 expression during early stages of
meiotic prophase [77]. In addition, an SCP3+
synapsis of two chromosomes was detected in human primordial follicle oocytes
(arrowhead, panel L and insert), possibly representing XX chromosomal
synapsis, as sex chromosomes start synapsis during early zygotene, before
autosomes synapse [77]. Rare SCP3+ oocytes (less
than 10%) were detected in midfollicular phase ovaries. The highest expression
of SCP3 (10 to 30% of primordial follicle oocytes) was found in postovulatory
ovaries during the early luteal phase in younger (up to 38 years of age) women.
However, at age 42, postovulatory ovaries showed no SCP3 expression. Virtually
no staining of oocytes was observed in three younger women studied during the
mid- and late luteal phases, or in polycystic ovaries [71].
Panel M shows SCP3 expression in adult rat testes (positive control). Note SCP3
immunostaining of condensed chromosomes in spermatogonia (red arrowhead) and
advanced progression of meiotic division in primary spermatocytes (black
arrowhead) in a 2-month-old rat male gonad.
These
observations indicate that SCP3 is expressed in adult human and monkey ovaries,
confirming that neo-oogenesis occurs in primates during adulthood. Preparation
for meiotic activity may have already occurred at the level of tunica albuginea
stem cells, and meiotic prophase activity may continue and terminate in oocytes
of newly formed follicles. As indicated by Kayisli and Seli: "If proven to occur
in human, the implications of de-novo oocyte formation from stem cells would be
significant for our understanding of fertility and our approach to its
preservation" [78].
Adult
rat ovaries
Studies
of human ovaries raise the question as to whether formation of new oocytes
exists in other adult mammalian species. We studied ovaries of adult rat
females by immunohistochemistry and found migrating ameboid germ cells [36] resembling the migrating germ cells found in adult
human ovaries [35], and clusters of dividing germ
cells expressing zona pellucida (ZP) proteins in unstained solid epithelial
cords. These observations indicated that germ cells, some of which exhibit the
ameboid shape, may develop in adult rat ovaries. These cells may originate from
the OSC. An alternative site is the ovarian hilar region, which contains sex
cords replete with bone morphogenetic protein (BMP) ligands and receptors [79].
Using a double staining
immunohistochemistry technique [72], we found
that bone marrow derived cells (MDC and T cells) are also involved in
triggering germ cell development from the OSC in adult rats. The MDC (not
shown) and T cells (black asterisk, Figure 6N) accompanied asymmetric
division of OSC giving rise to ZP+ germ cells (red vs. yellow asterisk). These descend
into the adjacent solid epithelial cord, also described in ovaries of adult
guinea pigs [80], a source of granulosa cells
under the OSC layer. Large oogonia divided symmetrically (crossing over) in the
solid epithelial cords (Figure 6O; see also [81]
for mice), and such division was accompanied by MDC (yellow arrowhead, panel P).
Blue arrowheads indicate association of solid epithelial cord cells
representing primitive granulosa cells. Note that in adult human ovaries
symmetric division of emerging germ cells is apparent (Figure 6E) and no
dividing oogonia were detected [35], while in
adult rat ovaries besides emerging germ cells [36],
the new oogonia can also symmetrically divide (panels O and P).
Divided
rat oogonia separated, and the resulting oocytes formed new primordial
follicles. Monocyte-derived cells also accompanied the growth of primordial
follicles. In adult rats lacking OSC after neonatal estrogen treatment, the
germ cells indeed originated in the ovarian hilar region (see above) and formed
primordial follicles in the juxtaposed (deep) ovarian cortex [72].
These
observations indicate that similar pathways of new oocyte development exist in
different mammalian species, although there may be variations in the routes of
granulosa cells contributing to the formation of new follicles. For example,
the availability of epithelial cell cords in adult rats resembles human fetal
ovaries [36]. In contrast, OSC in adult human
ovaries produce the cord cells which are very similar to some of the granulosa
cells. In some areas of the ovary, cords fragment and appear as small 'nests'
of epithelial cells. Typically, these epithelial nests (fragmented cords) lie
in proximity to primordial follicles [82,83].
Our
observations indicate that these primitive granulosa cell nests are descending
into the deep cortex where they assemble with vessels to catch circulating
oocytes or surround OSC crypts to assemble with migrating germ cells [35]. Hence in adult women, the number of granulosa
cell nests determines the number of newly formed follicles, since superfluous
new oocytes degenerate in medullary vessels. Accordingly, even if some new
oocytes form after the end of the prime reproductive period (PRP; women between
menarche and 38+
2 years of age - reviewed in [35,84]), the lack of developing granulosa cell nests precludes the
formation of new follicles. Due to the progressive diminution of the remaining
aging follicular pool, menopause occurs. Preliminary termination of either new
oocyte or granulosa cell nest formation results in premature ovarian failure [85].
Bone
marrow derived cells and the "storage" vs. a "prime reproductive
period" doctrines
In
1923, Edgar Allen [81] introduced a distinction
between the "storage" theory, which is based on the opinion that there may
never be any increase in the number of oocytes beyond those differentiating
during fetal or perinatal ovarian development [86],
versus the "continued formation" of oocytes theory, which suggests that
oogenesis is maintained throughout the life of mammals [35,57,71,80,81,87].
The
currently prevailing "storage" doctrine, as elaborated by Sir Solly
Zuckerman and collaborators (reviewed in [88]),
is based on the following milestones (assumptions): A)
Total number of oocytes declines with age by a simple regression.
B)
Oocytes persist in rat ovaries lacking ovarian surface epithelium (i.e. OSC).
C)
Oogonia do not persist in adult ovaries.
D)
Oogenesis from somatic stem cells is missing.
E)
Mitotic division of oogonia is missing.
Regarding
assumption (A), there is no significant decline during 20 years of reproductive
life, between 18-38 years of age in humans [89].
In addition, Faddy [90] indicated that the
pattern of primordial follicle number decline is not exponential, but more
bi-exponential corresponding to a 'broken-stick' regression of logged total
numbers of follicles against age. Such a model implies an abrupt change in the
exponential rate of follicle loss at age 38 years, and is thus rather
implausible biologically [90]. The model,
however, will be biologically plausible when follicular renewal is considered
to act before (slow decay rate during oocyte renewal) but not after 38 years of
age (fast decay rate during oocyte storage).
Regarding
the argument (B) that OSC are not essential for neo-oogenesis since the oocytes
persist in ovaries lacking OSC, we recently demonstrated that in rat ovaries
lacking OSC, the oocytes originate by an alternate pathway, from medullary
somatic stem cells; primordial follicles are formed in the juxtaposed (deep)
ovarian cortex [72].
Assumption (C) is in principle correct,
since the oogonia should not persist in adult ovaries, due to the threat of
accumulation of genetic anomalies with age. Yet, in adult human females,
precursors of germ cells are tunica albuginea stem cells [35], which have a mesenchymal character and are
certainly more resistant to environmental threats and to the accumulation of
genetic abnormalities with age. Differentiation of OSC from ovarian tunica
albuginea precursors is triggered by activated MDC [70].
Regarding
point (D), step by step oogenesis and follicular renewal from somatic stem
cells have been described in fetal and adult human and adult rat ovaries [35,36,57,72].
Finally,
regarding query (E), the mitotic division of newly formed germ cells and
oogonia has been described in human and rat ovaries [35,72].
Regarding
both the storage and continued oocyte formation paradigms, there appears to now
be a consensus that germ cells per se do not persist in adult mammalian ovaries
from the fetal/perinatal period. From the view of groups attempting to
re-establish the "continued formation" doctrine and search for the
origin of new germ cells in adult humans and laboratory rodents [35,57,72,91], there appears to be a consensus that
during adulthood the germ cells originate from progenitor cells. Two possible
mechanisms for the generation of new oocytes in postnatal mammals have been
recently proposed by Joshua Johnson [92].
1)
New oocytes are produced via germ stem cells that reside in an extragonadal
location, the bone marrow, and are released into the peripheral blood. These
progenitors migrate to the ovary, where they may engraft as new oocytes within
new follicles [91]. The developmental potential
of labeled oocytes after bone marrow transplantation remains unclear [93].
2)
New oocytes are produced by a transformative mechanism. Ovarian bipotential
progenitor cells produce both new oocytes and somatic (granulosa) cells within
the ovary [35,57,94].
More
recently, it has been reported that bone marrow transplantation improves
attenuated fertility after low dose chemotherapy in mice, although all newborns
were of recipient and not of bone marrow donor origin [95].
Tilly's group introduced in 2004 the idea of the origin of female germ cells in
mice from persisting germline stem cells in the ovary [96].
A year later, this was replaced with the idea of the extra ovarian origin of
mouse putative germ cells from bone marrow [91].
They also now found the idea on the origin of germ cells from bone marrow
untenable, suggesting that bone marrow cells function primarily by reactivating
host oogenesis impaired by chemotherapy [95].
However, they did not indicate which bone marrow cells are involved and how and
where the new germ cells originate in the recipient. Our studies suggested that
the bone marrow derived white blood cells (monocytes and T lymphocytes)
accompany the origin of new germ cells from OSC in fetal and adult human and
adult rat ovaries, or from medullary stem cells in adult rats lacking OSC
(reviewed in [72,84]). Furthermore, activated
resident vascular pericytes and bone marrow derived monocytes accompany
initiation of follicular growth, selection, and preovulatory maturation of
autologous oocytes [57,70,84]. We propose that
the lack of activated pericytes and bone marrow derived monocytes committed for
the stimulation of follicular growth and maturation of the allogeneic (donor)
oocytes may be why the primordial follicles formed from circulating donor germ
cells were found to be unable to differentiate and undergo ovulation [91,93,95].
During
certain periods of life, however, the storage of oocytes in mammals may occur.
Recently, we attempted to establish a harmony between the "storage"
and "continued formation" theories by proposing the "prime
reproductive period" theory [56,72,84,97]
as follows: the "storage" theory pertains to two periods of the life in human
females, that is between the termination of fetal oogenesis and puberty or
premenarcheal period (about 10 to 12 years), and premenopausal period following
the end of the PRP until menopause (also about 10 to 12 years). On the other
hand, the "continued formation" theory accounts for the follicular renewal
during the PRP (about 25 years, i.e., between menarche and 38+
2 years of
age), and ensures an availability of fresh oocytes for the development of
healthy progeny. Since the number of primordial follicles begins to diminish in
aging rodents [98], one may consider the
relevance of the PRP theory in these species as well.
In
conclusion, we are convinced that the neo-oogenesis and follicular renewal
during the PRP exists throughout the animal kingdom, including higher
vertebrates.
Vascular
pericytes and MDC regulate differentiation and selection of human ovarian
follicles
Within the adult human ovary, cohorts of
primordial follicles occupy distinct areas in the cortex (dashed line, Figure 7A),
characterized by diminution of Thy-1 expression in stromal cells [35,70] In these areas stromal cells show enhanced MHC
class I expression [57]. Most of the primordial
follicles remain in the resting state (rf, Figure 7A), but some show an
increase in size and apparent transformation into growing (secondary) follicles
(gf) accompanied by increased activity (Thy-1 release) of pericytes
(arrowheads, Figure 7B). This could be stimulated by due permissive
signals from innervation of follicular vessels (autonomic innervation +, Figure 1), since innervation controls quantity, but not quality of tissues and their
structures [32]. Initiation of follicular growth
is also associated with an interaction of pericytes (arrowheads, Figure 7B) and
activated macrophages (semi-parallel section, Figure 7C). Note HLA-DR+
material, an indicator of activated MDC [99],
secreted near granulosa cells and oocyte (arrowhead, Figure 7C), and
accumulating in the nuclear envelope of granulosa cells (black vs. white
arrows). Figure 7D (semiparallel section to B and C) shows strong MHC class I
expression, an indicator of epithelial cell differentiation (see Figure 2F),
and the cuboidal shape of granulosa cells, which accompanies this process.
![Selection of secondary (A-D)
and preovulatory (dominant) follicles (E-F) in the adult human
ovary. Staining for Thy-1, HLA-DR (DR), MHC class I light chain (β2m),
cytokeratin 18 (CK) and CD68 of mature MDC, as indicated in panels. Dashed
line in (A) indicates an area exhibiting diminution of Thy-1
expression by stromal cells. (B), detail from (A). (C)
and (D) are semi-parallel sections to (B). Dashed line in (E-J),
follicular basement membrane. rf, resting follicles; gf, growing follicle;
p, pericytes; e, endothelial cells; v, microvasculature in theca interna
(t); vl, vascular layer adjacent to the follicular basement membrane; g,
granulosa layer. Details in text. Adapted from Ref. [70],
© Wiley-Blackwell.](/article/100024/figure/F7/large)
Figure 7.
Selection of secondary (A-D)
and preovulatory (dominant) follicles (E-F) in the adult human
ovary. Staining for Thy-1, HLA-DR (DR), MHC class I light chain (β2m),
cytokeratin 18 (CK) and CD68 of mature MDC, as indicated in panels. Dashed
line in (A) indicates an area exhibiting diminution of Thy-1
expression by stromal cells. (B), detail from (A). (C)
and (D) are semi-parallel sections to (B). Dashed line in (E-J),
follicular basement membrane. rf, resting follicles; gf, growing follicle;
p, pericytes; e, endothelial cells; v, microvasculature in theca interna
(t); vl, vascular layer adjacent to the follicular basement membrane; g,
granulosa layer. Details in text. Adapted from Ref. [70],
© Wiley-Blackwell.
Usually
only one dominant follicle is selected for ovulation during the mid follicular
phase of each menstrual cycle in the human ovary. This process of follicular
selection still remains an unresolved puzzle. Premature stimulation with
gonadotropins results in multiple ovulations, suggesting that more than one
large antral follicle in the cohort developing up to the middle of the
follicular phase is capable of ovulating. Hence, under normal conditions, there
seems to be a competition among growing follicles themselves in an attempt to
reach the mature state and suppress the development of others. In contrast with
this traditional view, our data indicate that the follicles showing the most
advanced development during selection are not the dominant follicles. A
critical role in the process of dominant follicle selection appears to belong
to the theca interna compartment [29,100].
In
antral follicles of mammalian ovaries, including humans, two clear cut zones in
theca interna were detected. About one-third of the cells corresponding to a
more internal region (inner or vascular layer) were not stained with
luteinizing hormone receptor, 3-beta-hydroxysteroid dehydrogenase, and
P450-17alpha-hydroxylase antibodies. This contrasted with the remaining
two-thirds of cells corresponding to the external regions (outer or
steroidogenic layer), which were strongly labeled [101-103].
In dominant follicles, the inner vascular layer of theca interna contains
vascular pericytes secreting Thy-1 differentiation protein among
differentiating granulosa cells [27,29].
Figure 7E shows cytokeratin staining of a human dominant follicle in mid-follicular
phase with multiple granulosa cell layers (g) adjacent to the basement membrane
(dashed line). Under the follicular membrane is a vascular theca interna layer
(vl) surrounded by a steroidogenic theca interna layer (t) with narrow vessels
(v). Staining for Thy-1 (Figure 7G) shows that a high activity of Thy-1
pericytes is restricted to the vascular layer. The MDC releasing CD68 (Figure 7I)
are absent from the steroidogenic layer (arrows), but abundant in the vascular
layer.
Large antral follicles undergoing atresia
in the same ovary show detachment of granulosa cells from the basement membrane
(Figure 7F). This is accompanied by activation of pericytes in the
steroidogenic layer (white t, Figure 7H) and dilatation of vascular
lumina (v; compare panels F and H vs. E and G). In addition, the MDC in the
steroidogenic layer become highly activated (white arrowhead, Figure 7J), but
those in the vascular layer show low or no CD68 release (arrow). Instead, the
MDC from the vascular layer invade among granulosa cells (black arrowhead) [70].
Since
regulation of follicular selection involves autonomic innervation [59,60], we suggest that activation of pericytes in
the steroidogenic thecal layer of follicles undergoing atresia is caused by
permissive neuronal signals. On the other hand, retardation of pericyte
activity in a dominant follicle steroidogenic thecal layer during selection is
caused by a lack of such signals (autonomic innervation -, Figure 1).
Novel
aspects of follicular selection
Follicles
are selected twice during their development (secondary from primordial and
preovulatory from antral follicles), but the consequences for the remaining
follicles are different. First, during basal growth, secondary follicles are
selected from primordial follicles under the control of growth factors of
paracrine origin. Unselected primordial follicles remain in the resting state.
The selection of secondary follicles is associated with activation of pericytes
in adjacent micro-vasculature, possibly due to permissive signals from
autonomic innervation, which is involved in the regulation of quantitative
aspects (amounts) of specific cells and structures in tissues from early
periods of life [32,104]. This also causes an
activation of perivascular MDC. Hence, during growth initiation, the selected
follicles are stimulated in further development.
After
attaining the antral stage, follicles become gonadotropin dependent and
immature granulosa cells can be affected by thecal androgens [105]. Hence, premature acceleration of theca interna
steroidogenic layer development may cause follicular atresia by thecal
androgens via alteration of immature granulosa cells lacking aromatase. This is
associated with conversion of follicular MDC into phagocytes infiltrating the follicular
antrum. We show that during selection of pre-ovulatory follicle, the pericytes
in steroidogenic layer of theca interna in non-dominant follicles are highly
activated and accompanied by activated MDC. In contrast, non-activated MDC are
present in the vascular layer adjacent to the follicular basement membrane, and
invade the granulosa layer of non-dominant follicles. Hence, it appears that
the dominant follicle is selected by a process of temporary retardation of
steroidogenic thecal differentiation, possibly by a negative influence of
autonomic innervation on thecal pericytes. Extracts of the superior ovarian
nerve have been shown to inhibit thecal cell androstenedione production [106,107].
Once
the dominant follicle matures into the preovulatory stage, with the ability of
mature granulosa cells to convert androgens into estrogens [105], pericytes and MDC in both steroidogenic and
vascular layers of the dominant follicle show high activity [29,57]. Taken together, acceleration of steroidogenic
thecal layer development during follicular selection results in premature
androgen production causing detachment of immature granulosa cells from the
follicular basement membrane, invasion of macrophages into the follicular
antrum, and progression of atresia of non-dominant follicles [70].
Corpus
luteum
The
CL of the menstrual cycle has the shortest lifespan of any tissue structure in
the mammalian body. In women, its function ceases after two weeks, followed by
transformation into the amorphous corpus albicans. The association of various
types of immune cells with the CL during its development and regression
indicates that the immune system is involved in CL management. The absence of
the CL during early ontogeny, including immune adaptation, suggest that the CL
could be viewed by the immune system as a graft [17].
Although the ovary is densely innervated, with autonomic nerves associated with
thecal vessels of all follicles regardless of the stage of development, the
luteal vessels lack autonomic innervation [108].
Another feature of the CL is that, in contrast to some other tissues, such as
the liver, it is unable to regenerate. Moreover, during pregnancy, the CL can
survive and function for an extended period. This longevity is associated with
a change in behavior of luteal mesenchymal and immune cells. Thus the CL is a
unique model for the study of TCS mediated mesenchymal-epithelial interactions
without influence of innervation.
Figure 8A shows a young CL (2 days after the LH peak) with high activity of Thy-1
pericytes, characterized by secretion of intercellular vesicles (arrow) which
are converted into empty "spikes" (arrowhead). A mature CL (5 days
post ovulation) shows partial diminution of pericyte activity (Figure 8B). In
the CL of pregnancy (3rd month) the pericytes persist in an inactive state (Figure 8C). Regressing CL (early follicular phase of the next cycle) shows regression
of pericytes (Figure 8D) and infiltration by T cells (inset). This is
accompanied by IgG binding to luteal cells (not shown). Similar features were
observed during CL regression at the end of pregnancy. In the corpus albicans
no luteal cells are present and remnants of pericytes accompany regressing
microvasculature (Figure 8E).
The IgM distribution is shown in Figure 8F
to J. When compared to IgG, IgM is expressed phylogenetically (sharks) and
ontogenetically (immature B cells) much earlier [99].
Accordingly, IgM shows binding to tissue cells at various stages of their
differentiation. In the stratified epithelium IgM binds to the young
(parabasal) cells (IgM1), isolated mature and all aging (top of interstitial
layer) cells (IgM2), and a layer of the most superficial cells (IgM3) [3,51] - see also above. The IgM also binds to
endothelial cells, depending on the stage of differentiation of tissue cells.
![Staining for Thy-1, IgM, CD8,
and CD14, as indicated in panels, in human corpora lutea and ovarian
adenocarcinomas (OvCa). YCL, young CL; MCL, mature CL; CLP, CL of
pregnancy; RCL, regressing CL (subsequent follicular phase); CAlb, corpus
albicans. mv, microvasculature. Scale bar in E applies to panels A-O,
including insets. Details in text. Adapted from Ref. [109], © Elsevier.](/article/100024/figure/F8/large)
Figure 8.
Staining for Thy-1, IgM, CD8,
and CD14, as indicated in panels, in human corpora lutea and ovarian
adenocarcinomas (OvCa). YCL, young CL; MCL, mature CL; CLP, CL of
pregnancy; RCL, regressing CL (subsequent follicular phase); CAlb, corpus
albicans. mv, microvasculature. Scale bar in E applies to panels A-O,
including insets. Details in text. Adapted from Ref. [109], © Elsevier.
Figure 8F shows strong IgM binding to the granulosa lutein cells in the young
CL. Note a lack of binding to the endothelium of microvasculature (mv). In the
mature CL no IgM binding is apparent to either granulosa lutein or endothelial
cells (Figure 8G). The CL of pregnancy shows no IgM binding to granulosa lutein
cells, but strong binding to the vascular endothelial cells extended toward the
pericytes (Figure 8H). In regressing CL (Figure 8I), IgM binds to both
regressing luteal cells and vascular components. In the corpus albicans, IgM
binds to the amorphous structure and to residual microvasculature (Figure 8J).
Perivascular
primitive CD14 MDC show high activity (secretion of CD14 material into the
intercellular space) in the young CL (Figure 8K). This is accompanied by
secretion of CD68 and HLA-DR by MDC. The activity of MDC diminishes in the
mature and aging CL, where the MDC show a conversion into dendritic cells.
Subsequently, from the beginning of the next menstrual cycle, the luteal cells
show strong expression of various MDC and leukocyte markers, including CD14,
CD68, HLA-DR, leukocyte-common antigen, and CD4 of MDC and of helper T cells [17].
These
observations indicate that the TCS components vary with CL development,
preservation during pregnancy, and regression. High activity of vascular
pericytes and primitive MDC is characteristic for the CL development, and T
cells and dendritic cells accompany CL regression, resembling graft rejection.
OVARIAN CANCERS
Ovarian
cancers represent a wide variety of cell types with variable metastatic
potential. The most common types are adenocarcinomas, often expanding into the
peritoneal cavity and metastasizing to the omentum. Depending on the location
(primary vs. metastatic), and stage of differentiation (poor, moderate, well
differentiated), the activity of stromal and intraepithelial mesenchymal cells
in ovarian carcinomas varies. Figure 8L-O, shows several examples of
mesenchymal cell activity in various ovarian adenocarcinomas in addition to
those reported earlier [108,109].
Figure 8L shows high activity of Thy-1 pericytes in a poorly differentiated
ovarian adenocarcinoma. Note secretion of Thy-1 vesicles (arrow), the presence
of empty spikes among malignant cells (black arrowhead) and in the sprout of
endothelial cells (white arrowhead). Such activity is similar to that seen in
the young CL (see Figure 8A). However, pericytes in well differentiated
adenocarcinomas show low or no activity, similar to that observed in the
persisting CL of pregnancy.
IgM
binding is restricted to the microvasculature (Figure 8M), and none of the 20
adenocarcinomas investigated showed IgM binding to proliferating (Ki67+) or
differentiating malignant cells. This situation is also similar to the
persisting CL of pregnancy.
The most common feature seen in ovarian
cancers was the high activity of MDC. Figure 8N shows secretion of CD14
from immature MDC into the malignant epithelium (see also young CL in Figure 8K).
Similar activity was observed in CD68 and HLA-DR monocyte-derived cells [109]. A proportion of adenocarcinomas (9/20) showed
infiltration of malignant cells by T lymphocytes. Panel O shows normal T cells
within the malignant stroma (black arrow). T cells which enter differentiating
malignant epithelium release CD8 material among malignant cells (white arrow).
Deeper within the tumor, the T cells become smaller and exhibit low CD8
expression (arrowheads in inset), features characteristic of their apoptotic
fragmentation [4,108].
MDC
in ovarian epithelial inclusion cysts and pro-inflammatory cytokines in ovarian
cancers
Epithelial
inclusion cysts (EICs), are formed by trapping of ovarian surface epithelium
(OSE) cells within the ovarian stroma during ovulation wound repair or ovarian
surface inflammatory processes. It is widely accepted that EICs constitute a
preferential site of ovarian carcinogenesis. OSE cells in EICs undergo
Müllerian metaplasia and acquire the architectural and functional characteristics
of the epithelia of Müllerian duct derivatives, such as Fallopian tube,
endometrium or endocervix. Tubal metaplasia, the most common differentiation
pathway in EICs, is characterized by the appearance of secretory and ciliated
cells (arrowheads, Figure 9A and C) and expression of specific genes such as
CA125 and oviductal glycoprotein [110]. Notably,
differentiation of OSE cells in EICs through Müllerian pathways is
associated with the presence of monocyte derived CD68 positive cells (MDC) that
infiltrate the cyst wall and accumulate in the cyst lumen (arrows, Figure 9A
and B) [111]. MDC are a source of active
cytokines that could reach bioactive concentrations in the confined space of
the EICs, thus affecting the differentiation and proliferative activity of
epithelial cells (Figure 9C) contributing to the initial stages of OSE cell
transformation.
The
levels of pro-inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6 and TNF-alpha
were higher in ovarian cancerous tissues than in normal specimens [112,113]. The higher levels of these factors were
detected mainly in epithelial cells of the tumor than in the surrounding
stromal cells (Figure 9D and E).
Altogether,
there might be novel strategies for the targeting activity of MDC in
"tissue control units" associated with cancer [109].
Figure 8N shows association of primitive CD14 MDC with growing malignant
cells. CD14 is a lipopolysaccharide receptor [114],
which inhibits activation of NK cells capable of recognizing and killing tumor
cells [115]. It is possible that temporary
targeting of CD14 MDC, e.g. by CD14 antibody [109],
small interfering (si)RNA [116], or other
approaches targeting tissue macrophages, may result in the regression of
unstable (lack of autonomic innervation) "tissue control units" in
malignant tissues, leading to activation of NK cells, and subsequent regression
of malignant stroma and elimination of tumor cells.
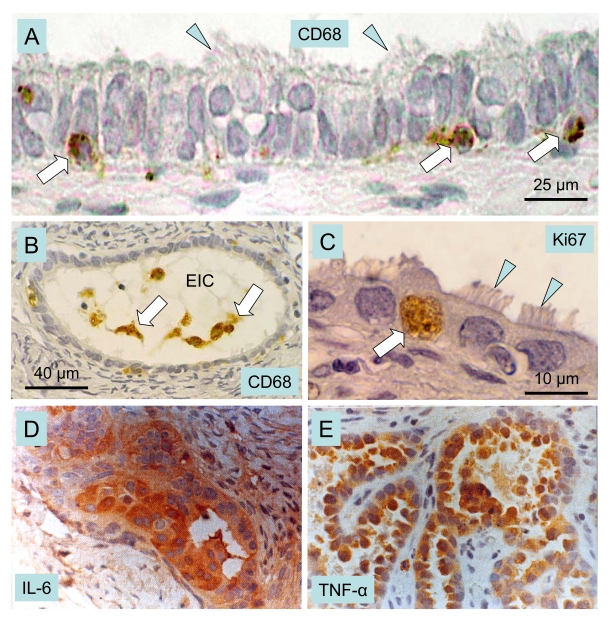
Figure 9. Macrophages, cytokines and ovarian cancer.
Epithelial inclusion cysts (EIC), showing infiltration of
the cyst wall (A) and lumen (B) by CD68 positive MDC
(arrows), ciliated cells (arrowheads), and Ki67 positive (arrow in C)
proliferating cells. Immunohistochemical staining of cancerous ovarian
tissues for IL-6. (D) and TNF-alpha (E) (x400). A-C adapted
from Ref. [111], © Elsevier, and D and E from
Ref. [113], © John Libbey Eurotext Ltd.
These data indicate that malignant growth
is associated with enhanced activity of mesenchymal cells, and tissue
macrophages in particular. Novel approaches to cancer prevention and control
may depend on a better understanding of the mechanisms by which tissue
macrophages promote growth of tumor cells. In addition, studies of events
accompanying regression of luteal tissue may be of importance for better
understanding on how the regression of vascular components results in ultimate
regression of epithelial/parenchymal and possibly malignant tissues [70].
IMMUNE ADAPTATION AND THE DETERMINATION OF FUNCTIONAL
TISSUE LIFESPAN
During
immune adaptation (through the end of the second trimester of intrauterine life
in humans [99]), differentiating tissues are
recognized by the developing lymphoid (immune) system as self [117-119]. However, depending on the time point at
which a certain tissue arises during immune adaptation, cellular memory can
determine how long MDC and T cell support will persist. In the ovary, these
cells influence formation of new germ and granulosa cells and differentiation
of primordial follicles [36,57].
In
normal adult individuals, the first organ affected by aging is the thymus [120], and the next are the ovaries [121,122]. There is a correlation between the period
at which an organ is present during early ontogeny and its functional
longevity. For instance, the heart, which differentiates very early, can
function in humans for over one hundred years. In contrast, the ovaries, which
differentiate later, do not function for more than half that time (Figure 10A).
We have proposed that the later the differentiation of certain tissues occurs
during early ontogeny, the earlier its function expires during adulthood [31]. Ovarian development is influenced by
mesenchymal-epithelial interactions which accompany the emergence of germ cells
and follicular growth [4,57,70]. Uncommitted
MDC may first recognize and memorize the character of OSC, which differentiate
from urogenital coelomic epithelium populated by primordial germ cells. In the
fetal ovary, presumptive memory cells reside in the rete ovarii, and
uncommitted MDC and T cells migrate through rete channels toward the ovarian
surface and participate in the development of germ cells from the OSC [36]. Similar interaction of immune cells with OSC was
described in the ovaries of adult women [57].
During adulthood, however, no rete is present in ovaries, so the memory cells
may reside in the lymphoid tissues, the source of antigen-committed immunocytes
[99]. The immune system shows a significant
functional decrease between 35 and 40 years of age in women [123], and concomitantly ovarian follicular renewal
wanes [35].
Premature
ovarian failure (POF) could be caused by delayed ovarian development during
immune adaptation (SHORTER, Figure 10A), by earlier termination of immune
adaptation, or by cytotoxic chemotherapy affecting both the existing pool of
primordial follicles and the OSC committed bone marrow-derived cells (T cells
in particular) required for the emergence of new secondary germ cells and hence
for follicular renewal. Patients with POF have been found to have abnormalities
in the function of circulating monocytes, activated lymphocytes, and NK cells,
and exhibit other immune abnormalities [124-126],
suggesting a relationship between immune system and POF.
![Immune adaptation and TCS
"stop effect." (A) Immune adaptation (IA) and tissue
longevity. The heart differentiates from early stages of ontogeny (LONG IA)
and functions throughout life. The ovary differentiates later (MODERATE
IA), and its normal function is limited by follicular renewal (until 35-40
years of age). Aging primordial follicles (apf) persist until exhausted
(physiologic menopause). SHORTER period of ovarian development during IA
causes earlier termination of follicular renewal during adulthood and
results in POF. SHORT period of ovarian development during IA causes no
follicular renewal and results in primary amenorrhea. Absence of corpora
lutea (CL) during immune adaptation causes their cyclic degeneration,
except during pregnancy, which is accompanied by immune suppression. fpf,
fetal primordial follicles; fr, follicular renewal; POF, premature ovarian
failure; CL, corpus luteum. Adapted from Ref. [31].
(B) Stages of cell differentiation during immune adaptation (left)
sets TCS "stop effect" (StE) for tissue physiology and pathology
during adulthood. Arrowheads indicate a tendency to StE "shifts" with age.
Adapted from Ref. [3,30,33,108].](/article/100024/figure/F10/large)
Figure 10. Immune adaptation and TCS
"stop effect." (A) Immune adaptation (IA) and tissue
longevity. The heart differentiates from early stages of ontogeny (LONG IA)
and functions throughout life. The ovary differentiates later (MODERATE
IA), and its normal function is limited by follicular renewal (until 35-40
years of age). Aging primordial follicles (apf) persist until exhausted
(physiologic menopause). SHORTER period of ovarian development during IA
causes earlier termination of follicular renewal during adulthood and
results in POF. SHORT period of ovarian development during IA causes no
follicular renewal and results in primary amenorrhea. Absence of corpora
lutea (CL) during immune adaptation causes their cyclic degeneration,
except during pregnancy, which is accompanied by immune suppression. fpf,
fetal primordial follicles; fr, follicular renewal; POF, premature ovarian
failure; CL, corpus luteum. Adapted from Ref. [31].
(B) Stages of cell differentiation during immune adaptation (left)
sets TCS "stop effect" (StE) for tissue physiology and pathology
during adulthood. Arrowheads indicate a tendency to StE "shifts" with age.
Adapted from Ref. [3,30,33,108].
Thymus
and reproduction
The
thymus plays an important role in the immune system, and it has been suggested
that thymic peptides participate in determining the reproductive lifespan of
females [127,128]. The relationship between
age-associated thymic involution and diminution of ovarian function is
evidenced by the alteration of ovarian function in neonatally thymectomized
mice [129]. In congenitally athymic (nude) mice,
follicular loss is first evident at 2 months of age, specifically due to a
reduction in the numbers of primordial follicles. The first ovulation is
delayed until two and a half months of age, compared to one and half months in
normal mice. By four months, an overall reduction in all fractions of the
follicle population occurs in nude mice, and ovulation ceases [130].
THE TISSUE CONTROL SYSTEM AND A
"STOP-EFFECT" OF MDC THEORY
By
the end of immune adaptation in early ontogeny, the MDC are proposed to
encounter the most differentiated cells in a specific tissue, and prevent them
from differentiating beyond the encoded state during adulthood by the so called
"stop effect." The power of this "stop effect" may reside
in the inability of monocyte-derived cells to stimulate differentiation of
tissue cells beyond the encoded stage [3].
Retardation or acceleration of differentiation during immune adaptation may
cause a permanent alteration of tissue function. If the ability of monocytes to
preserve tissue cells in a functional state declines with age, a functional
decline would ensue, leading to menopause and degenerative diseases.
In
large mammals including primates immune adaptation ceases during intrauterine
life, while in laboratory rodents (rats and mice) immune adaptation continues
for several postnatal days, ending about one week after birth [99]. Estrogens given to neonatal rats or mice inhibit
ovarian development, and during adulthood females show persisting ovarian
immaturity characterized by a retardation of follicular development [30] despite normal serum levels of gonadotropins [131,132]. This indicates that suppression of early
ovarian development results in persisting ovarian immaturity, which resembles
POF associated with the gonadotropin resistance of ovarian follicles. Injection
of estrogens in neonatal mice (days 0-3) caused permanent anovulation, but mice
injected later (days 3-6; closer to the end of immune adaptation) showed
resumption of ovulatory cycles after initial anovulation [133]. Hence, persisting ovarian immaturity can result in
a delay of normal ovarian function. Since the incidence of degenerative
diseases increases with age, one may expect the "stop-effect" to
shift with age (arrowheads, Figure 10B). This could explain why an immature
ovary may switch to a functioning ovary.
On
the other hand, injection of androgens causes premature ovarian aging which
persists during adulthood. However, androgen induced anovulation may be
prevented by neonatal injection of a thymic cell suspension from
immunocompetent prepubertal normal female donors, unless the animal donors did
not complete immune adaptation [134,135]. This
suggests that certain thymic cells (thymocytes, or thymic MDC) of normal
immunocompetent females carry information about appropriate differentiation of
ovarian structures, and this information can be transferred to immunologically
immature neonatal rats. Hence the state of tissue differentiation during immune
adaptation determines tissue function in adult individuals.
When
a lower dose of androgens is injected during immune adaptation, the rats
exhibit a so-called delayed anovulatory syndrome. Ovaries exhibit the onset of
normal function after puberty (~40 days of age), but premature aging of the
ovary occurs between 60-100 days [136]. This
delayed manifestation of ovarian dysfunction resembles human POF with secondary
amenorrhea as well as some human degenerative diseases with an autoimmune
character. The latter similarly occur after a shorter (juvenile diabetes
mellitus) or longer (Alzheimer's disease) period of normal tissue function.
A
simplified view of the TCS theory of the regulation of tissue function via the
"stop effect" (StE) is depicted in Figure 10B (see
also [4,17,34]). In normal tissues, the mature cells are present
during immune adaptation, and the tissue-specific cells are "parked"
in the mature state during adulthood. Retardation of cell differentiation
during adaptation results in persisting immaturity (POF with primary
amenorrhea) and acceleration in premature aging (POF with secondary amenorrhea,
degenerative diseases). If the tissue is absent during adaptation (e.g. corpus
luteum), it will be handled immunologically as a "graft."
IMMUNE PHYSIOLOGY AND REGENERATIVE MEDICINE
Totipotency of ovarian stem cells in vitro
Ovarian stem cells exhibit a totipotent
character resembling embryonic stem cells, since they are able to differentiate in vitro into oocytes, parthenogenetic embryos, and neural/neuronal cell
types, [94,137,138] and OSC cultures exhibit
markers of embryonic stem cells [139,140].
![Oocyte and parthenote
development in vitro. (A) The oocyte development in OSC
culture is accompanied by a satellite (black arrow) and neuronal (white
arrow) cells. White arrowhead indicates neuronal extension. (B) DAPI
staining of (A). (C) The parthenote shows a blastocoele
(white arrow) and inner cell mass (black arrow). (D) DAPI staining
of (C). Four cell embryo. (E - G) and morula (H and I).
J and K panels show a blastocyst consisting of blastocoele
(bc), trophectoderm (te), and inner cell mass (icm) releasing ESC (esc).
Left insert in panel K shows enhanced DAPI staining of dividing ESC
vs. low DAPI staining of other cells in the culture (right insert). Details
in text. Adapted in part from Ref. [137], ©
Cambridge Journals.](/article/100024/figure/F11/large)
Figure 11. Oocyte and parthenote
development in vitro. (A) The oocyte development in OSC
culture is accompanied by a satellite (black arrow) and neuronal (white
arrow) cells. White arrowhead indicates neuronal extension. (B) DAPI
staining of (A). (C) The parthenote shows a blastocoele
(white arrow) and inner cell mass (black arrow). (D) DAPI staining
of (C). Four cell embryo. (E - G) and morula (H and I).
J and K panels show a blastocyst consisting of blastocoele
(bc), trophectoderm (te), and inner cell mass (icm) releasing ESC (esc).
Left insert in panel K shows enhanced DAPI staining of dividing ESC
vs. low DAPI staining of other cells in the culture (right insert). Details
in text. Adapted in part from Ref. [137], ©
Cambridge Journals.
Advanced
differentiation of oocytes in vitro produced oocytes of 200 μm
diameter (Figure 11A) - note the wide ZP and compare with the small size of surrounding
cells. The oocyte was accompanied by satellite cells (black arrow) substituting
for granulosa cells in providing additional resources (organelles) needed by
the developing oocyte [141]. Also involved were
neuronal type cells (white arrow) with an extension (arrowhead) expanding over
the oocyte. Panel B shows DAPI staining - note a large oocyte nucleus. Some
oocytes differentiated into parthenogenetic embryos with extensively developed
blastocoels (white arrows, panels C and D) and inner cell mass (black arrows).
Deleted
azoospermia like (DAZL) protein was strongly expressed in early parthenotes
(arrowhead, Figure 11E), at the four cell stage (panels F and G).
Resulting morulae (panels H and I; no immunohistochemistry) can develop into
blastocysts that show production of DAZL+ embryonic stem cells (ESC) from the
embryonic inner cell mass into the culture (arched arrow, panel J). The inner
cell mass and the released ESC are mitotically active as compared to the other
cells lacking DAZL expression and pronounced DAPI staining (left vs. right
inset, panel K) [97,137].
These
observations indicate that OSC cultures could be a source of oocytes for the
treatment of female ovarian infertility, and can also produce ESC for the
purposes of autologous regenerative medicine.
Epithelial
to neural/neuronal transition is triggered by a mixture of sex steroids
Within
the field of regenerative medicine of neurodegenerative and traumatic
neurologic diseases, there is considerable interest in cellular therapy, such
as grafting of neural stem cells (NSC) into the CNS in order to induce neuronal
renewal and repair of degenerative, traumatic or ischemic defects. Neural stem
cells can be isolated from the neonatal or adult CNS and propagated in vitro in the presence of mitogenic growth factors prior to use [142,143]. Alternative sources of NSC are ESC,
umbilical cord blood, amniotic epithelial cells, bone marrow stem cells, and
mobilized peripheral blood CD133+ cells [144-147].
After several passages, these cells can be transdifferentiated into NSC either
by fibroblast growth factor-1, 12-otetradecanoylphorbol-13-acetate (protein
kinase C activator), isobutyl-methylxanthine (a non-specific inhibitor of
phospho-diesterases), and forskolin (protein kinase A activator), or by
all-trans-retinoic acid and 2-mercaptoethanol [148].
These substances are not suitable for treatment in vivo, however. Previous work
from our laboratory demonstrated that occasionally, neuronal cells
spontaneously appear in cultures of human ovarian epithelial stem cells [94].
An alternative to the use of organ-tissue specific stem cells for functional grafting to particular sites (topical
therapy) could be a "systemic regenerative treatment" with
utilization of common drugs with a low molecular weight.
Sex steroids may have the potential to stimulate the proliferation and
differentiation of existing NSC. They easily pass the blood-brain barrier and
can bind to abundant sex steroid receptors in the brain areas important for the
regulation of emotions, cognition, and behavior [149].
However it still remains to be determined whether utilization of individual sex
steroids alone might be efficient in prevention or treatment of
neurodegenerative diseases and traumatic neurologic injuries. To address this
question, we studied the effects of sex steroids on the transdifferentiation of
totipotent human ovarian epithelial stem cells and porcine granulosa cells into
NSC and neuronal cells [138].
Human ovarian epithelial stem cells differentiated
into large epithelial cells lacking expression of stage specific embryonic
antigen (SSEA) -1 (Figure 12A) and neural cell adhesion moleculae (NCAM; not
shown). A few epithelial cells in untreated cultures showed moderate staining
for Thy-1 (panel B) and similar expression of SSEA-4 (not shown). Addition of
individual gonadotropins, EGF, or sex steroids alone showed no change in either
cell morphology or immune-histochemical staining. No changes were observed in
control cultures including those with the sex steroid vehicle.
On
the other hand, utilization of testosterone (TS) mixed with progesterone (PG)
one day after estradiol (E2) pretreatment produced a marked effect after one
hour. There was transdifferentiation of epithelial cell clusters into small
cells, a portion of which strongly expressed SSEA-1 glycoconjugate of NSC and
precursor cells [150] and others were less
densely SSEA-1 positive (black vs. solid grey arrowheads, panels C and D). An
asymmetric division resulting in SSEA-1+ and SSEA-1- daughter cells is shown in
panel E; note stained early extensions (arrow) associated with the SSEA-1+
cell. Many of the nascent small cells strongly expressed Thy-1, a GPI-anchored
protein abundantly expressed by neurons [151].
Particularly strong expression was apparent in cells detaching from the
moderately stained cluster of cells (black vs. solid grey arrowheads, panel F).
The detached cells, however, still showed connections (arrows) with the cell
cluster. More distant cells exhibited development of Thy-1+ extending processes
(left black arrowhead), suggesting early stages of neuronal differentiation.
Some larger cells with developed extensions strongly expressed NCAM (black
arrowhead, panel G), which is characteristic of later stages of neuronal
differentiation [150]. Antibody to NCAM is used
for the isolation of human ESC-derived neurons [150].
Less developed (smaller) cells
in the cluster showed moderate NCAM expression only (solid grey arrowhead).
![Human ovarian epithelial stem
cell cultures (representative images from four experiments): untreated (A and B); pre-treated for 1 day with E2 and 1h after TP+PG treatment (C-G);
pre-treated for 1 day with E2 and 3h after TS+PG treatment (H-O).
Lack of SSEA-1 expression (A) and moderate Thy-1 expression by some
epithelial cells (B). SSEA-1 is strongly expressed in some small
cells resembling stem cells (black vs. white arrowheads, C and D)
and one of the cells originating by asymmetric division (E). Similar
cells show strong expression of Thy-1 (F). The NCAM expression was
also detected in some cells (G). Two hours later, the cells reached
neuronal morphology and exhibited SSEA-1 expression in the cell bodies but
not extending processes (H, black vs. white arrowheads), Thy-1 and
NCAM expression in both (I and J, black arrowheads), and
SSEA-4 expression slightly exceeding that of SSEA-1 (K vs. H).
No staining was observed in the immunohistochemistry control (L).
Panels M-O show phase contrast microscopy with neuronal and
epithelial cells (M), floating numerous putative NSC (N), and
putative NSC exhibiting bubble type anchors (arrowheads, O). Numbers
above bars indicate microns. For details see text. Adapted from Ref. [138], © Landes Bioscience.](/article/100024/figure/F12/large)
Figure 12. Human ovarian epithelial stem
cell cultures (representative images from four experiments): untreated (A and B); pre-treated for 1 day with E2 and 1h after TP+PG treatment (C-G);
pre-treated for 1 day with E2 and 3h after TS+PG treatment (H-O).
Lack of SSEA-1 expression (A) and moderate Thy-1 expression by some
epithelial cells (B). SSEA-1 is strongly expressed in some small
cells resembling stem cells (black vs. white arrowheads, C and D)
and one of the cells originating by asymmetric division (E). Similar
cells show strong expression of Thy-1 (F). The NCAM expression was
also detected in some cells (G). Two hours later, the cells reached
neuronal morphology and exhibited SSEA-1 expression in the cell bodies but
not extending processes (H, black vs. white arrowheads), Thy-1 and
NCAM expression in both (I and J, black arrowheads), and
SSEA-4 expression slightly exceeding that of SSEA-1 (K vs. H).
No staining was observed in the immunohistochemistry control (L).
Panels M-O show phase contrast microscopy with neuronal and
epithelial cells (M), floating numerous putative NSC (N), and
putative NSC exhibiting bubble type anchors (arrowheads, O). Numbers
above bars indicate microns. For details see text. Adapted from Ref. [138], © Landes Bioscience.
Three
hours after the TS+PG mixture following E2 pretreatment (panels H-O), the cells
showed an advanced neuronal morphology with mutually connected extending
processes, suggesting what we term "brain in vitro" features.
Stage-specific embryonic antigen-1 expression was limited to neuronal cell
bodies, and their extending processes were unstained (black vs. white
arrowheads, panel H). The neuronal cells strongly expressed Thy-1 glycoprotein
in both the cell bodies and extending processes (black arrowheads, panel I),
and the same applied for NCAM expression (panel J). Stage-specific embryonic
antigen-4 is commonly used as a cell surface marker to identify pluripotent human
ESC and expressing cells enriched in the neural stem/progenitor cell fraction [152]. It was strongly expressed in neuronal cell
bodies but virtually absent in extending processes (black vs. white arrowhead,
panel K). Control immunohistochemistry produced no staining of neuronal (white
arrowhead, panel L) or other cell types. In phase contrast observations, the
neuronal phenotype cells were shown to interact with remaining epithelial cells
(panel M). Large numbers of putative NSC detached from the bottom of the flasks
and were found floating in the center of the wells (panel N). These cells
showed bubble type anchoring extensions (arrowheads, panel O), which apparently
serve to attach seeded non-neuronal cells [138].
Since such putative NSC did not attach again, they may be ready for homing
where needed.
In
summary, OSC cultures could produce oocytes suitable for in vitro fertilization
[153], and the treatment can be complemented by
implantation of fertilized oocytes in the uterus regardless of the type of
ovarian failure. However, regenerative medicine for neurodegenerative and
traumatic neurologic diseases depends on local TCS interactions in the given
tissue in the particular individual. For instance local or systemic
implantation of NSC in Alzheimer's disease does not preclude that such cells
will differentiate into mature neurons, if the brain lacks neurosteroids and
MDC-derived microglia, supposed to be required for such a process. Moreover,
even when such substances and cells are available, the differentiating neurons
may not be preserved in a functional state and may continue to degenerate due
to the lack of a proper "stop-effect" (see Figure 10B). Proper conditions can
be, however, expected in the traumatic neurologic diseases, where regenerative
medicine or systemic regenerative treatment may result in a more favorable
outcome. Recently, NSC derived from human ESC have been shown to engraft
functionally in an experimental mouse model with an improvement of sensimotor
function of the stroke-disabled forelimb [154].
The
authors declare that they have no competing financial interests.