Requirement of DDR-related factors in DDR of EC
To investigate the requirement of DDR-related factors in DDR of EC, we generated flies with EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, and Chk2, which are sensors, mediators, or effectors of the DDR system, using flies with the Myots>GFP genotype. DDR directs a cell to repair DNA double-strand breaks (DSBs), a major driver of intrinsic aging. γH2AX is a dependable indicator of DNA damage response [50,51]. To determine the activation of DDR in ECs when exposed to DNA damage, we examined the signal strength of γH2AvD, analogous to mammal γH2AX, in Myo-GFP+ cells of the gut from Myots>GFP flies 1 h after the application of 5 Gy of γ-ray irradiation as an inducer of the DNA damage. While week γH2AvD signals were detected in ECs and Myo-GFP- cells (ISCs, EBs, and EEs) in the non-irradiated Myots>GFP wild-type flies (Fig. 1A a-a’, yellow arrow), strong γH2AvD signals were detected in ECs and in Myo-GFP- cells (ISCs, EBs, and EEs) in the irradiated Myots>GFP wild-type flies (Fig. 1A i-i’, yellow arrow). This indicates the activation of DDR in EC against DNA damage. To determine the requirement of DDR-related factors in DNA damage-induced DDR activation in ECs, we examined the signal strength of γH2AvD in Myo-GFP+ cells of the gut from Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies 1 h after irradiation. In contrast to the signal in wild-type Myots>GFP flies, the γ-irradiation-induced increase in the γH2AvD signal was greatly reduced in Myo-GFP+ cells (ECs) of Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies (Fig. 1A j-p’, yellow arrow). At this time point, strong γH2AvD signals were detected in Myo-GFP- cells (ISCs, EBs, and EEs) from the irradiated Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies (Fig. 1A j-p’). These results indicated that the EC-specific knockdown of DDR-related factors specifically affected the activation of DDR system in ECs.
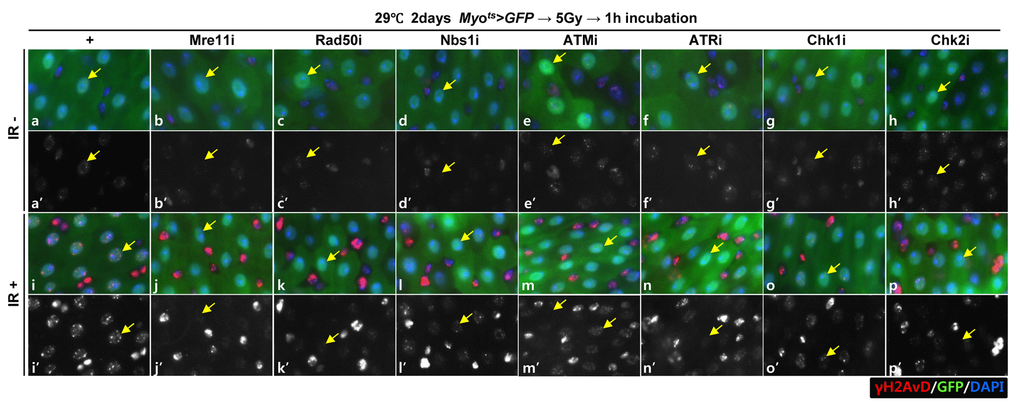
Figure 1A. EC-specific knockdown of DDR cause EC cell death. Effect of knockdown of EC-specific DDR-related factors on γH2AvD signals after irradiation. γH2AvD signals after 5 Gy irradiation in the EC-specific Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2 knockdown in the midgut. Flies carrying Myots>GFP (a-a’ and i-I’), Myots>GFP+Mre11i (b-b’ and j-j’), Myots>GFP+Rad50i (c-c’ and k-k’), Myots>GFP+Nbs1i (d-d’ and l-l’), Myots>GFP+ATMi (e-e’ and m-m’), Myots>GFP+ATRi (f-f’ and n-n’), Myots>GFP+Chk1i (g-g’ and o-o’), or Myots>GFP+Chk2i (h-h’ and p-p’) were cultured at 29 °C for 2 days and exposed to γ-radiation. a-h’, non-irradiation. i-p’, 5 Gy irradiation. a-p panels depict the merged images and a’-p’ panels denote the gray scale versions for the images corresponding to γH2AvD signals. One hour after irradiation, the guts of the irradiated flies were dissected and labeled with anti-GFP (green) and anti-γH2AvD (red) antibodies and 4′,6-diamidino-2-phenylindole (DAPI, blue). Yellow arrows indicate EC. Original magnification is 400×.
Knockdown of DDR-related factors in the EC induces EC death
To assess the role of DDR-related factors in EC death, we examined Cleaved caspase-3 signals and found that they were increased in the Myo-GFP+ cells of the gut from Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies kept at 29 °C for 4 days. Very weak Cleaved caspase-3 signals were detected in ECs in Myots>GFP wild-type flies (Fig. 1B a-a’). In contrast to the signal in the wild-type Myots>GFP flies, the Cleaved caspase-3 signal was greatly increased in Myo-GFP+ cells (ECs) of Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies (Fig. 1B b-h’). We quantified the ratio of Cleaved caspase-3+ in Myo-GFP+ cells. Significant increases of EC death were detected in the gut of EC-specific DDR-related factor knockdown (Fig. 1B i). In the Myo-GFP- small cells of the gut from Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i, signals of Cleaved caspase-3 were not detected (Fig. 1B). EC-specific DDR knockdown-induced ECs death could be suppressed by coexpression of the Caspase inhibitor, DIAP1 (Suppl. Fig. 1 and 2), indicating that DDR knockdown induced ECs death.
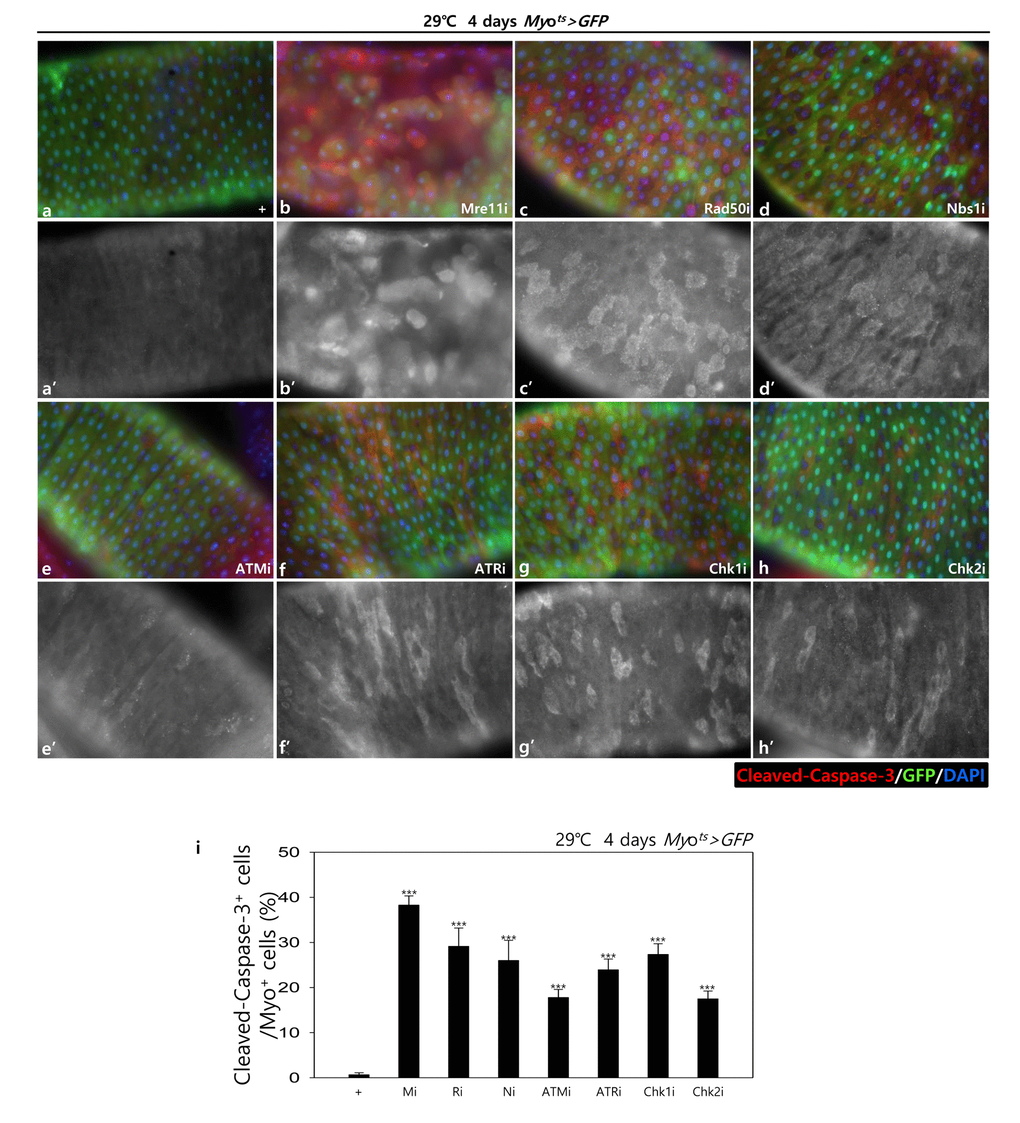
Figure 1B. EC-specific knockdown of DDR cause EC cell death. EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2 induce Cleaved caspase-3 in EC. Flies carrying Myots>GFP (a-a’), Myots>GFP+Mre11i (b-b’), Myots>GFP+Rad50i (c-c’), Myots>GFP+Nbs1i (d-d’), Myots>GFP+ATMi (e-e’), Myots>GFP+ATRi (f-f’), Myots>GFP+Chk1i (g-g’), or Myots>GFP+Chk2i (h-h’) genotypes were cultured at 29 °C for 4 days. a-h panels depict the merged images and a’-h’ panels denote the gray scale versions for the images corresponding to Cleaved caspase-3 signals. Original magnification is 400×. (i) A graph showing the ratio of Cleaved caspase-3+ cells in Myo-GFP+ cells. The data (mean ± SE) from 5 guts, respectively. ***p < 0.0001. The guts of flies were dissected and labeled with anti-GFP (green) and anti-Cleaved caspase-3 (red) antibodies and DAPI (blue).
In addition, to check the role of DDR-related factors on the activation of the JNK signal in EC cells, pJNK signals were examined in Myo-GFP+ cells of the gut from Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies kept at 29 °C for 4 days. Very weak signals of pJNK, a cell death marker, were detected in ECs in Myots>GFP wild-type flies (Fig. 1C a-a’). By contrast, the pJNK signal was greatly increased in the Myo-GFP+ cells (ECs) of Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies (Fig. 1C b-h’). We quantified the ratio of pJNK+ in Myo-GFP+ cells. The significant increases of EC death were detected in the gut of EC-specific DDR-related factor knockdown (Fig. 1C i). These results indicated that DDR-related factors are required for EC survival in normal conditions.
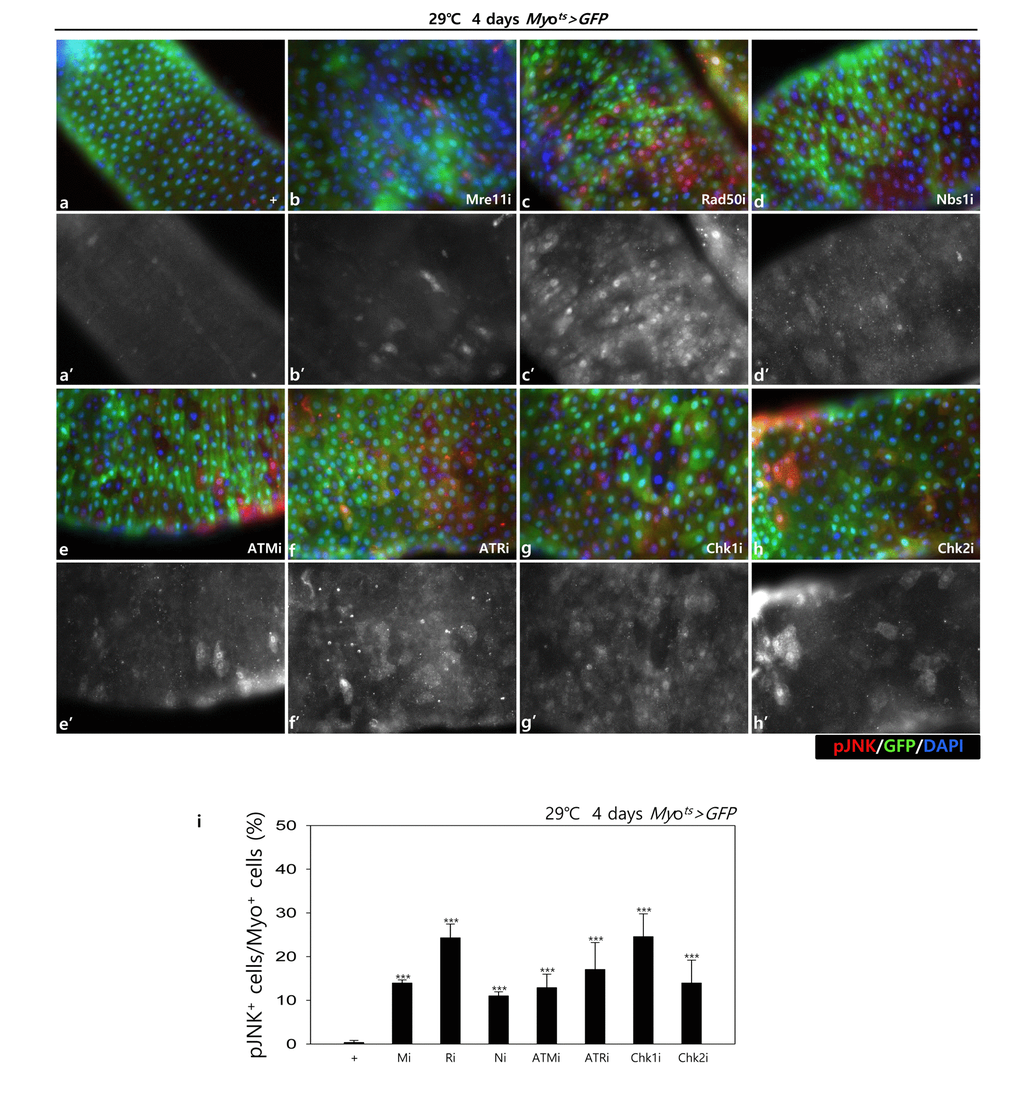
Figure 1C. EC-specific knockdown of DDR cause EC cell death. EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2 induce JNK activation in ECs. Flies carrying Myots>GFP (a-a’), Myots>GFP+Mre11i (b-b’), Myots>GFP+Rad50i (c-c’), Myots>GFP+Nbs1i (d-d’), Myots>GFP+ATMi (e-e’), Myots>GFP+ATRi (f-f’), Myots>GFP+Chk1i (g-g’), or Myots>GFP+Chk2i (h-h’) genotypes were cultured at 29°C for 4 days. a-h panels depict the merged images and a’-h’ panels denote the gray scale versions for the images corresponding to pJNK signals. Original magnification is 400×. (i) A graph showing the ratio of pJNK+ cells in Myo-GFP+ cells. The data (mean ± SE) from 5 guts, respectively. ***p < 0.0001. The guts of flies were dissected and labeled with anti-GFP (green) and anti-pJNK (red) antibodies and DAPI (blue).
Knockdown of DDR-related factors in EC induces ISC aging
Furthermore, we examined whether the knockdown of DDR-related factors in the EC affects ISC proliferation using anti-PH3 (a marker of mitotic cells) and anti-Delta (a marker of intestinal stem cell) antibodies. These gene knockdowns were assessed in ECs using Myots>GFP flies kept at 29 °C for 4 days. As expected, a dramatic increase in ISC proliferation was detected in the guts of Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies compared with that in the control (Fig. 2A). The number of PH3+ cells significantly increased in guts harboring the EC-specific knockdown of DDR-related factors (Fig. 2B). In addition, the number of Delta+ cells also dramatically increased in guts with EC-specific knockdown of DDR-related factors (Fig. 2C). These results indicated that the loss of DDR-related factors in ECs induced ISC hyperproliferation.
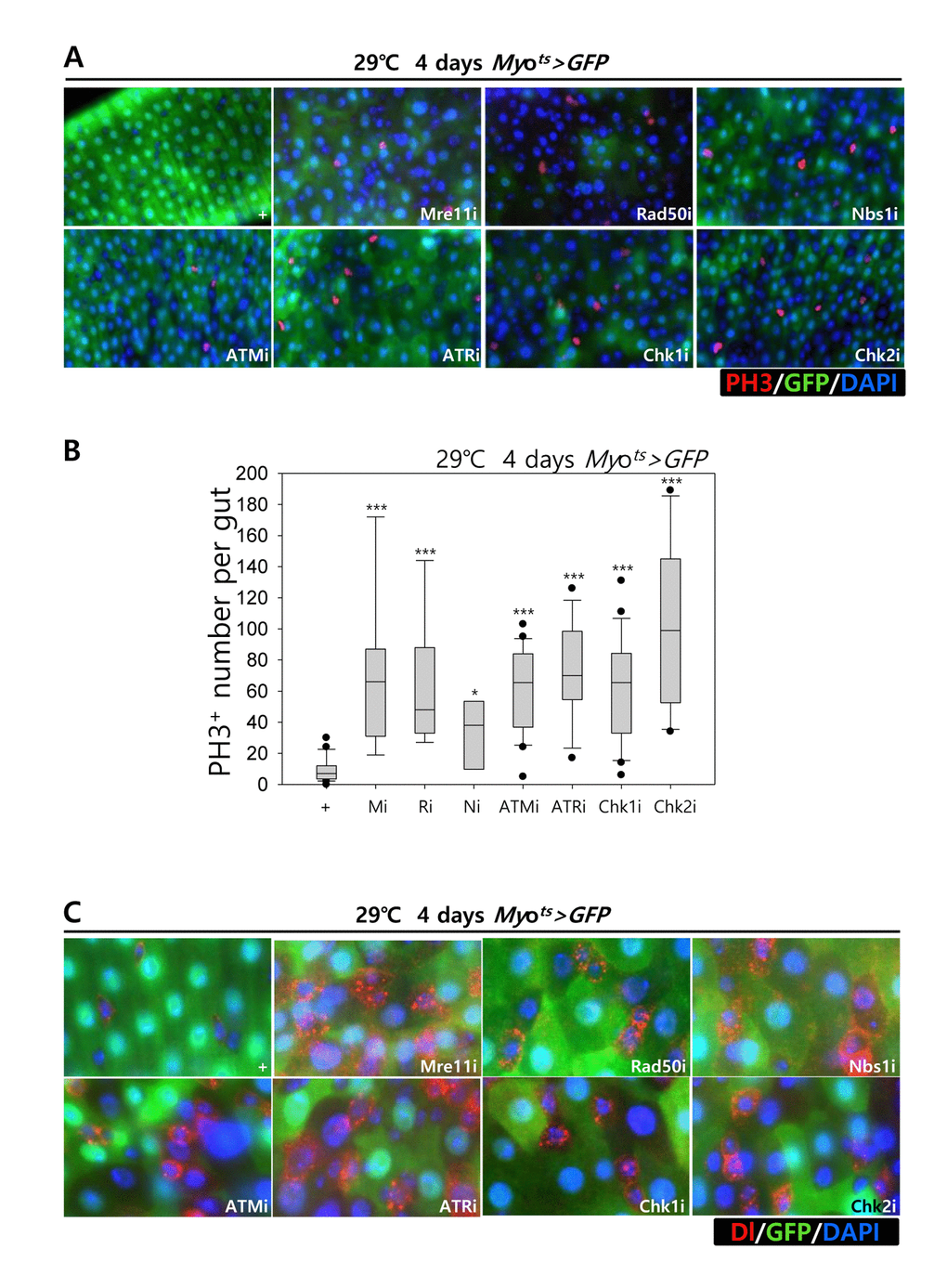
Figure 2. Effects of the knockdown of EC-specific DNA damage response (DDR)-related factors on ISC proliferation. (A-B) EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2 induce ISC division. Flies carrying Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i genotypes were cultured at 29 °C for 4 days. The guts of flies were dissected and labeled with anti-GFP (green) and anti-PH3 (red) antibodies and DAPI (blue). Original magnification is 400×. (B) A graph showing the PH3+ cell number in the midgut with an EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2. The gut specimens of Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i flies (kept at 29 °C for 4 days) were labeled with anti-GFP (green) and anti-PH3 (red) antibodies and DAPI (blue). The numbers of PH3+ cells were counted in the whole gut under a microscope. Data (mean±SE) in Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i flies were collated from 21, 22, 13, 20, 9, 9, 26, and 10 guts, respectively. p-values were calculated using student’s t-test. *p < 0.01, ***p < 0.0001. (C) EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2 increased the number of Delta-positive cells. Flies carrying Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i genotypes were cultured at 29 °C for 4 days. The guts of flies were dissected and labeled with anti-GFP (green) and anti-Delta (red) antibodies and DAPI (blue). Original magnification is 400×.
To assess the implication of excessive ISC proliferation by EC-specific DDR-related factor knockdown-induced EC death, we analyzed the DNA damage accumulation in ISCs using an anti-γH2AvD antibody, a molecular marker of the DSBs [14,52], and anti-Delta antibody. The γH2AvD signal was very low in the Myo-GFP- and Delta+ cells (a marker of ISCs) of Myots>GFP flies (Fig. 3A a-a’); however, γH2AvD foci were dramatically increased in the Myo-GFP- and Delta+ cells (ISCs) of Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies (Fig. 3A b-h’). These results indicated that the EC-specific knockdown of DDR-related factors could induce DNA damage accumulation in ISCs. Furthermore, EC-specific DDR knockdown-induced DNA damage accumulation in ISCs could be suppressed by coexpression of the DIAP1 (Suppl. Fig. 3), indicating that EC-specific DDR knockdown-induced ISC aging is associated with EC death. We also checked the centrosome amplification (a hallmark of cancer cells) using anti-γ-tubulin and anti-PH3 antibodies. In control files, two centrosomes in the mitotic ISCs (PH3+ cells) were detected; however, mitotic ISCs with 3–12 abnormal centrosomes were detected in the EC-specific DDR-related factor knockdown flies carrying Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i genotypes (Fig. 3B a). We quantified the frequencies of these mitotic ISCs with supernumerary centrosomes (>2), which were 9.4% in the Myots>GFP+Mre11i flies (N = 15, n = 449, N indicates the number of guts, n indicates the number of PH3+ cells), 12.2% in the Myots>GFP+Rad50i flies (N = 11, n = 557), 6.8% in the Myots>GFP+Nbs1i flies (N = 15, n = 412), 14.6% in the Myots>GFP+ATMi flies (N = 13, n = 560), 8.7% in the Myots>GFP+ATRi flies (N = 16, n = 447), 9.6% in the Myots>GFP+Chk1i flies (N = 15, n = 687), 13.2% in the Myots>GFP+Chk2i flies (N = 9, n = 349), and 1.6% in the Myots>GFP flies (N = 15, n = 61) (Fig. 3B c). The number of mitotic ISCs with supernumerary centrosomes (>2) per gut was 2.8 in the Myots>GFP+Mre11i flies, 6.2 in the Myots>GFP+Rad50i flies, 1.9 in the Myots>GFP+Nbs1i flies, 6.3 in the Myots>GFP+ATMi flies, 2.4 in the Myots>GFP+ATRi flies, 4.4 in the Myots>GFP+Chk1i flies, 5.1 in the Myots>GFP+Chk2i flies, and 0.07 in the Myots>GFP flies (Fig. 3B d). These results show that the inhibition of DDR resulted in DNA damage accumulation and in a higher incidence of centrosome amplification in ISCs.
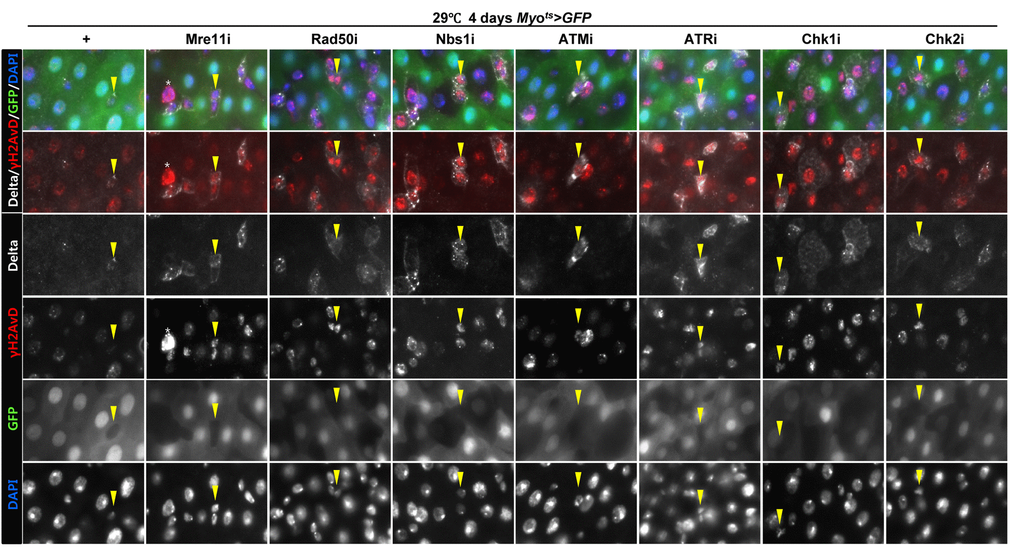
Figure 3A. EC-specific knockdown of DNA damage response (DDR)-related factors causes an increase in the age-related phenotypes of ISCs. EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2 induce DNA damage accumulation in ISCs. Flies carrying Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i genotypes were cultured at 29 °C for 4 days. The guts of flies were dissected and labeled with anti-GFP (green), anti-Delta (white), and anti-γH2AvD (red) antibodies and DAPI (blue). Yellow arrow heads indicate Delta+ cell. Upper two panels is merged image. Lower four panels is gray scale image of upper images. Asterisk indicates Myo-, Delta-, and strong γH2AvD+ cell, shows dying cell.
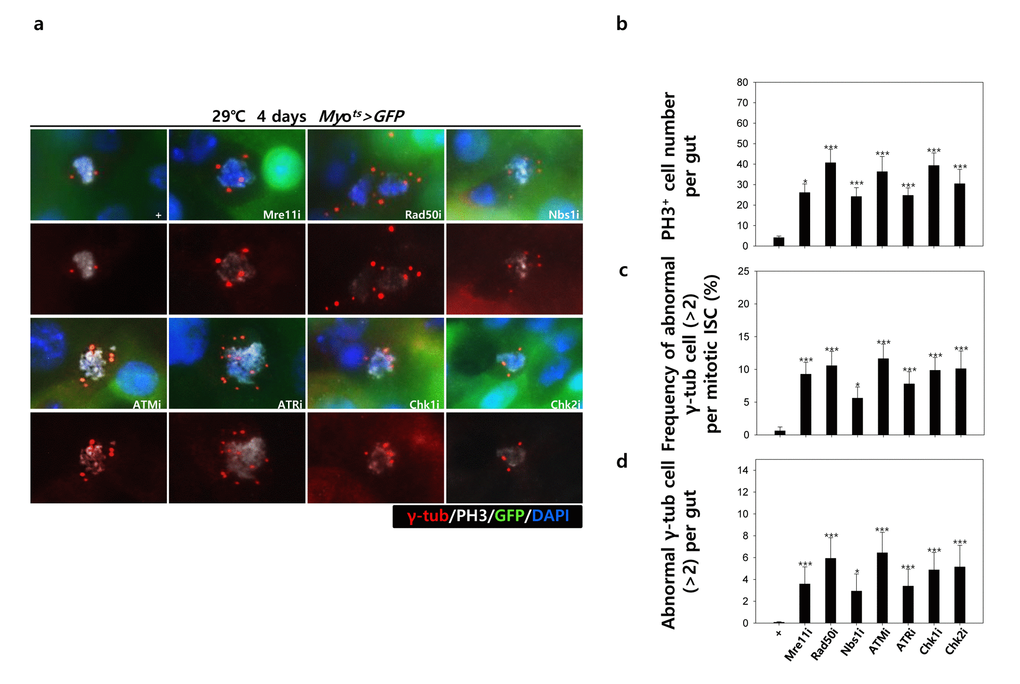
Figure 3B. EC-specific knockdown of DNA damage response (DDR)-related factors causes an increase in the age-related phenotypes of ISCs. EC-specific knockdown of DDR-related factors cause centrosome amplification in ISCs. Flies carrying Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i genotypes were cultured at 29 °C for 4 days. (a) The guts of flies were dissected and labeled with anti-GFP (green), anti-γ-tubulin (red), and anti-PH3 (white) antibodies and DAPI (blue). Original magnification is 400×. (b-d) Increased number of mitotic ISCs with supernumerary centrosomes (>2) in the guts of Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i flies. (b) EC-specific knockdown of Mre11, Rad50, Nbs1, ATM, ATR, Chk1, or Chk2 cause the increase of mitotic ISCs in the midguts. (c) Frequency of abnormal γ-tubulin cell per mitotic ISC. (d) Number of abnormal γ-tubulin cell per midgut. Three-day-old females were shifted to 29 °C for 4 days and dissected guts were immunostained with anti-GFP (green), anti-γ-tubulin (red), and anti-PH3 (white) antibodies and DAPI (blue). The centrosome numbers were counted in the PH3+ cells of these guts. Data (mean±SE) in Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i flies were collated from 61, 449, 557, 412, 560, 447, 687, and 349 mitotic cells of 15, 15, 11, 15, 13, 16, 15, and 9 guts, respectively. p-values were calculated using student’s t-test. *p<0.001, ***p<0.0001 compared to that of the Myots>GFP flies.
Collectively, the results indicated that the knockdown of EC-specific DDR-related factors induced age-related phenotypes of ISCs, ISC hyperproliferation, DNA damage accumulation, and a higher incidence of centrosome amplification.
Effect of knockdown of DDR-related factors in ECs at the organismal level
To further investigate the role of DDR-related factors at the organismal level, we checked whether EC-specific knockdown of DDR-related factors affected the adult fly’s survival. In the early stage of life, the survival of Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i females were significantly reduced compared to that of Myots>GFP females (Fig. 4A). Moreover, we assessed whether EC-specific DDR knockdown affected the response of ISCs to mild stress using an anti-PH3 antibody (a marker of dividing cells). Under 2 mM paraquat (PQ) fed conditions, ISC proliferation was highly increased in the guts of Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, and Myots>GFP+Chk2i flies, while it did not change in the guts of Myots>GFP flies (Fig. 4B). These results indicated that the guts with EC-specific knockdown of DDR-related factors are more sensitive to mild stress compared with that of wild-type flies.
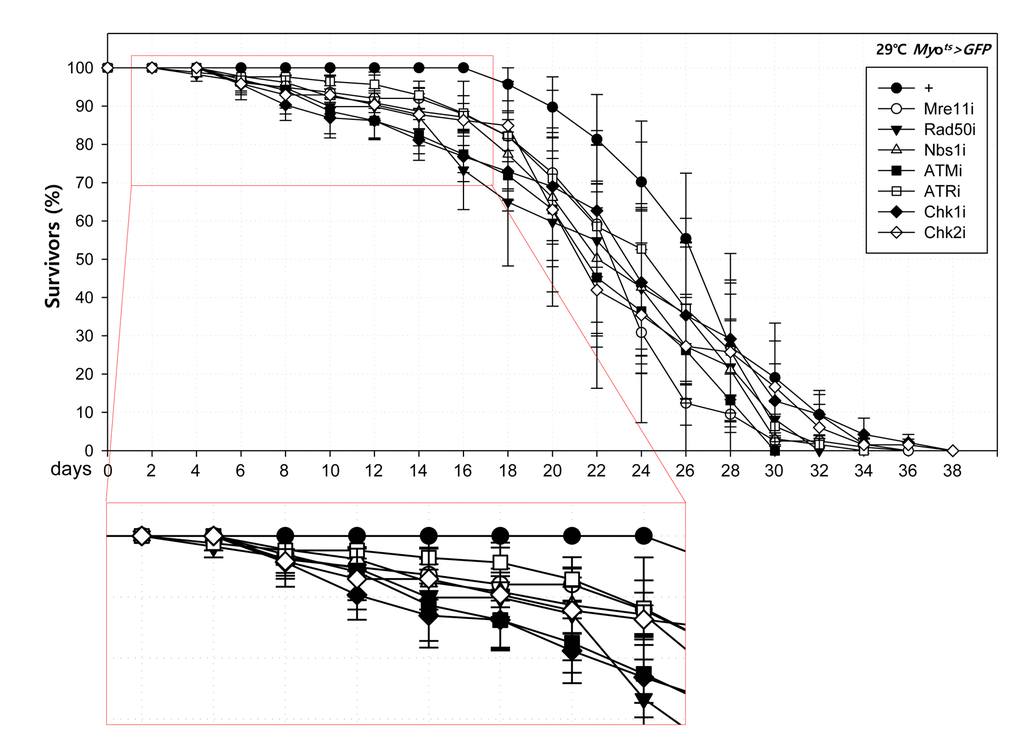
Figure 4A. High sensitivity to mild oxidative stresses exhibited by flies with EC-specific knockdown of DNA damage response (DDR)-related factors. Death rate at the early stage of flies with the knockdown of EC-specific DDR-related factors. Flies carrying Myots>GFP (closed circle), Myots>GFP+Mre11i (open circle), Myots>GFP+Rad50i (closed inverted triangle), Myots>GFP+Nbs1i (open triangle), Myots>GFP+ATMi (closed quadrangle), Myots>GFP+ATRi (open quadrangle), Myots>GFP+Chk1i (closed rhombus), or Myots>GFP+Chk2i (open rhombus) genotypes were cultured at 29 °C and survivors were counted every two days (n=62, 82, 56, 58, 77, 92, 78, 59, respectively).
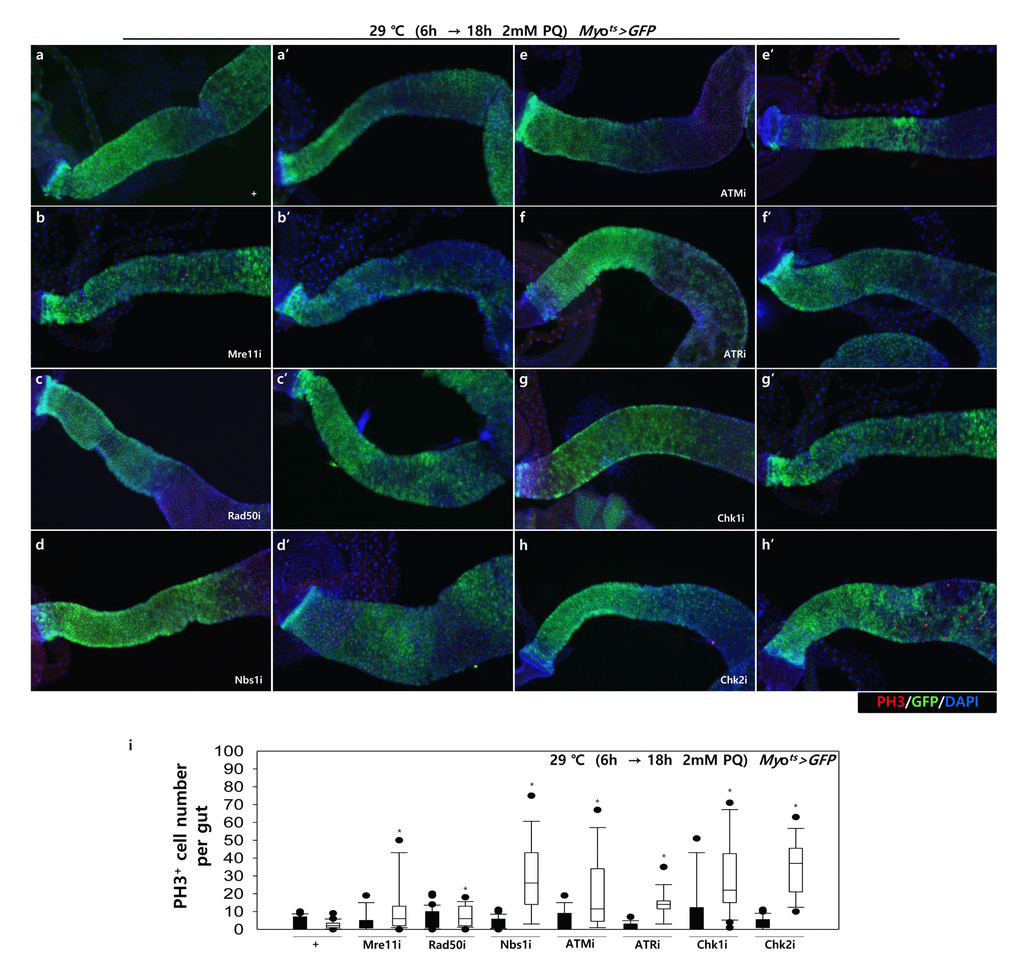
Figure 4B. High sensitivity to mild oxidative stresses exhibited by flies with EC-specific knockdown of DNA damage response (DDR)-related factors. EC-specific DDR-related factor knockdown flies evince a higher sensitivity to mild oxidative stress. Three-day-old flies carrying Myots>GFP, Myots>GFP+Mre11i, Myots>GFP+Rad50i, Myots>GFP+Nbs1i, Myots>GFP+ATMi, Myots>GFP+ATRi, Myots>GFP+Chk1i, or Myots>GFP+Chk2i genotypes were cultured for 6 h at 29 °C in normal media, and then were fed without (a-h, closed bars) or with 2 mM PQ (a’-h’, open bars) in 5% sucrose for 18 h at 29 °C. The number of PH3+ cells in their guts was imaged (a-h’) and counted (i). p-values were calculated using Student’s t-test. *p < 0.05.