Synthesis and degradation of PAR in the extracts
Considering the known role of the system of PAR synthesis/degradation in cell response to DNA damage and DNA repair, we compared the processes of PAR synthesis and degradation in four WCEs. The total level of PAR synthesis was evaluated by two approaches based on the use of [32P]NAD+ as a substrate. In one way, the aliquots of the reaction mixtures were loaded onto Whatman 1 paper. The paper-bound radioactivity level after the removal of unreacted NAD+ reflects the total amount of PAR synthesized by endogenous PARPs. An example of the kinetics of PAR synthesis is shown in Figure S1 A and B. The linear parts of the kinetic curves of this synthesis were observed until 1.5 min for HEK293T WCE and at least for 3 min for rodent WCE. It is important of note a large difference in the rate of PAR synthesis in the extracts of human and rodent cells. This fact prompted us to evaluate the efficiency by an additional approach.
Alternatively, the level of PAR synthesis by endogenous PARPs was analyzed by gel electrophoresis. The autoradiograph of the gel demonstrates an example of the analysis of PAR synthesized in WCEs and by PARP1 (Figure S1C). Quantification of the PAR yield in the extracts is shown as a bar chart in Figure 1A. The efficiency of PAR synthesis differs less than 3 times between the rodent cell extracts, with the maximum yield being observed in Hgl WCE. At the same time, the yield of PAR in HEK293T WCE is one order of magnitude higher than that in rodent cell extracts.
![Efficiency of PAR synthesis (A) and degradation (B) in WCEs. (A) PAR synthesis was performed for 1 min at 37 °C in the reaction mixture containing standard buffer components and 0.6 A260/mL activated DNA, 0.5 mg/mL cell extract proteins (or 10 nM recombinant human PARP1), and 20 μM [32P]NAD+. The reaction mixtures were treated and analyzed as described in the section ‘Synthesis and degradation of PAR in the extracts. PARP activity assay’. The yield of PAR analyzed by SDS-PAGE (the gel is shown in Fig. S1) is represented as a bar chart in arbitrary phosphorimager units. The analysis of PAR synthesis for three independent experiments is shown in numerical form under the bar chart. The data are the mean ± SD. In each experiment, the amount of PAR synthesized in the extract was normalized to that synthesized by 10 nM recombinant PARP1. (B) The reaction mixtures containing standard components, [32P]PAR synthesized as described in the section ‘Synthesis and degradation of PAR in the extracts. PARP activity assay’, and 0.5 mg/mL cell extract proteins or 10 nM recombinant PARG were incubated at 37 °C for different time intervals. Aliquots were further processed and analyzed as described in the section ‘Synthesis and degradation of PAR in the extracts. PAR degradation assay’. The amount of [32P]PAR in an equal aliquot of the control mixture (no proteins added) before incubation was taken as 100%. The points on the experimental curves represent the average of three independent experiments. Standard deviation did not exceed 10%.](/article/101959/figure/f1/large)
Figure 1. Efficiency of PAR synthesis (A) and degradation (B) in WCEs. (A) PAR synthesis was performed for 1 min at 37 °C in the reaction mixture containing standard buffer components and 0.6 A260/mL activated DNA, 0.5 mg/mL cell extract proteins (or 10 nM recombinant human PARP1), and 20 μM [32P]NAD+. The reaction mixtures were treated and analyzed as described in the section ‘Synthesis and degradation of PAR in the extracts. PARP activity assay’. The yield of PAR analyzed by SDS-PAGE (the gel is shown in Fig. S1) is represented as a bar chart in arbitrary phosphorimager units. The analysis of PAR synthesis for three independent experiments is shown in numerical form under the bar chart. The data are the mean ± SD. In each experiment, the amount of PAR synthesized in the extract was normalized to that synthesized by 10 nM recombinant PARP1. (B) The reaction mixtures containing standard components, [32P]PAR synthesized as described in the section ‘Synthesis and degradation of PAR in the extracts. PARP activity assay’, and 0.5 mg/mL cell extract proteins or 10 nM recombinant PARG were incubated at 37 °C for different time intervals. Aliquots were further processed and analyzed as described in the section ‘Synthesis and degradation of PAR in the extracts. PAR degradation assay’. The amount of [32P]PAR in an equal aliquot of the control mixture (no proteins added) before incubation was taken as 100%. The points on the experimental curves represent the average of three independent experiments. Standard deviation did not exceed 10%.
The replacement of activated DNA as a cofactor for endogenous PARPs of the extracts by the SP-BER substrate decreased the yield of PAR only by 20% in all extracts (data not shown). The efficient activation of PAR synthesis in the extracts by the BER substrates gives grounds to further study the impact of PARylation on DNA synthesis on these substrates.
To evaluate the rate of PAR degradation in the extracts, we used the following approach. Radioactively labeled PAR was synthesized by human recombinant PARP1. PAR synthesis was stopped by EDTA and the reaction mixture was used as a source of PAR without purification.
Poly(ADP-ribose) glycohydrolase (PARG) is the main enzyme responsible for PAR degradation in mammalian cells [25]. PARG acts as both endo- and exoglycosidase, which releases PAR of different length and ADP-ribose monomers and does not require bivalent metal ions for its activity [25]. The kinetic curves of PAR hydrolysis are shown in Figure 1B. In the negative control (no extract proteins added) the level of PAR decomposition for 30 min was less than 5% (data not shown). Again HEK293T WCE was the most active. In the case of PAR degradation, however, the rates between the extracts differed less than two fold.
The found difference in the activity of the PAR synthesis/degradation system between the WCEs makes them a convenient model system to evaluate a contribution of these processes to the regulation of DNA synthesis in the SP- and LP pathways of BER.
Effect of (ADP-ribosyl)ation on DNA synthesis
In the previous paper, we have already compared DNA synthesis in the specifically prepared extracts of mouse and naked mole rat fibroblasts on the BER substrates, however, the effect of PARylation on DNA synthesis was not addressed [1]. In contrast to the previous study, we used here the whole-cell extracts without fractionation of proteins (including the extract of human cells) and three BER substrates. They contain a nick and have the common upstream primer bearing the [32P]-label at the 5ʹ end, while the downstream oligonucleotides have either the deoxyribose phosphate (dRP) or diethylene glycol phosphate (pDEG) residues at the 5ʹ ends or the single-stranded dangling flap. The 5ʹ dRP moiety was obtained after the removal of the uracil residue by Ung, which led to the formation of the substrate of the initial stage of SP pathway. The DNA duplex containing the pDEG residue at the 5ʹ end of a downstream oligonucleotide mimics the substrate of the initial stage of the LP pathway since the pDEG residue cannot be removed by Polβ in contrast to the 5ʹ-dRP group. The flap-containing DNA can be considered as a product of the strand-displacement DNA synthesis and represents the substrate of the later stages of the LP pathway.
The results on DNA synthesis in the extracts are shown in Figure 2A. The tendency of the primer elongation characteristic for the particular BER substrate was similar in all extracts. The quantitative evaluation of product distribution according to their lengths is shown in Figure 2C (bottom row of diagrams). Taking into account the percentage of non-elongated primers and primers extended by one and several nucleotides, one can arrange the extracts according to the efficiency of DNA synthesis in the following order: HEK293T > Hgl > Mmu ≥3T3. It is worth noting that recombinant Polβ was the least efficient in the incorporation of the first nucleotide on the Flap substrate as compared to other BER substrates, while in HEK293T WCE comparable efficiency for all substrates was observed (Figure S2). This fact testifies to the direct involvement of the other cell extract proteins along with Polβ in DNA synthesis.
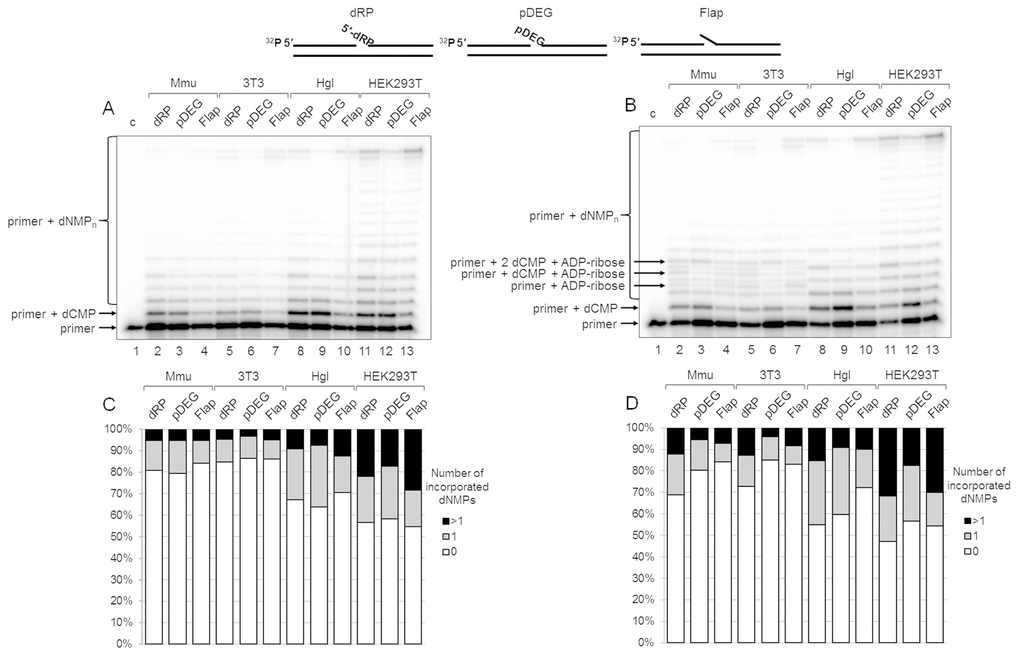
Figure 2. DNA synthesis and effect of PAR synthesis in WCEs. (A) DNA synthesis in the absence of NAD+. The cell extract proteins (0.5 mg/mL) were incubated for 5 min with 100 nM DNA duplexes bearing dRP, pDEG, or flap in the presence of 0.1 mM dNTPs (as described in the section ‘DNA synthesis assay’). (B) DNA synthesis in the presence of NAD+. The same as in (A), but in the presence of 0.5 mM NAD+. The unknown products are marked. Lanes 1 in A and B correspond to the initial primer (control). The types of DNA and cell lines are indicated. (C and D) Quantification of the products shown in Figure 2A and 2B, respectively. The white parts of the bars correspond to the non‐elongated primer, the grey parts reflect the amount of the primer elongated by one dNMP, and the black parts correspond to the products of strand‐displacement DNA synthesis. The intensity of the products is calculated as a percentage of the total radioactivity in the lane. The structures of DNA substrates are schematically shown at the top.
We intended to assess the influence of PAR synthesis on the efficiency of DNA synthesis in the presence of NAD+ in the extracts that differ significantly in the overall efficiency of PARylation. The data clearly demonstrate that the overall efficiency of DNA synthesis slightly increases in all cases irrespectively of the extract (compare Figure 2A with Figure 2B and in Figure 2C, top and bottom rows of diagrams). Generally, the effect of NAD+ was rather weak. In most cases, the amount of non-elongated initial primer decreased by less than 10% in the presence of NAD+ as compared with that in the absence of NAD+. However, the difference for the SP-BER substrate was slightly more pronounced in all extracts.
Interestingly, unusual products were formed in the presence of NAD+ in 3T3 and Mmu WCEs and in trace amounts in Hgl WCE. These products (marked in Figure 2B) were not registered in the absence of NAD+, and they were not characteristic for HEK293T WCE. We hypothesized that the unusual products appeared due to (ADP-ribosyl)ation of the primer during DNA synthesis. The possibility of PARP-dependent (ADP-ribosyl)ation of the terminal phosphate groups in DNA duplexes containing a gap/nick was demonstrated earlier [18–21], although under experimental conditions that did not involve DNA synthesis. The hypothesis of the primer (ADP-ribosyl)ation during DNA synthesis is confirmed by several experiments.
For convenience, mono(ADP-ribosyl)ation and poly(ADP-ribosyl)ation of an oligonucleotide to be elongated during the DNA synthesis is designated as MARylation and PARylation of a primer, respectively.
First, the products of (ADP-ribosyl)ation were not formed in the presence of olaparib, a PARP inhibitor, in the reaction mixtures with Mmu WCE (Figure 3A, compare lanes 2, 6, and 10 with lanes 4, 8, and 12). Second, the yield of the unusual products was significantly reduced in the presence of extra exogenous PARG (Figure 3A, compare lanes 2, 6, and 10 with lanes 5, 9, and 13), which is able to remove the ADP-ribose modification from DNA [21]. Third, the products of the same electrophoretic mobility appeared after incubation with mouse WCE and with mono(ADP-ribosyl)transferase PARP3 in the presence of NAD+ (Figure 4A, lanes 1 and 12, Figure 4B, lanes 2 and 9). Fourth, the indicated products are also formed in the reconstructed system containing Polβ and PARP3 instead of extract proteins (Figure 3B). Thus, our results together with the previously published data [19–21] allow for consideration of the unusual products as the MARylated primers containing the modifying group attached to the 5ʹ-terminal [32P]-phosphate group.
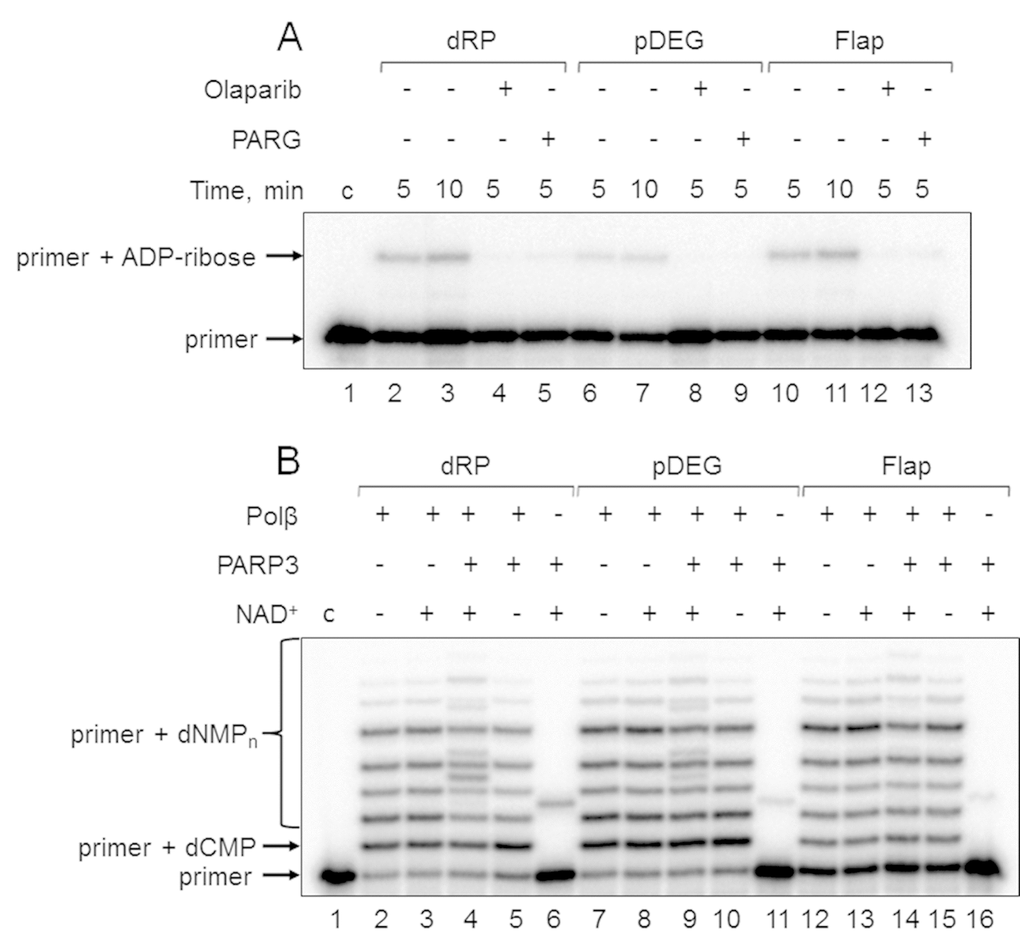
Figure 3. Demonstration of primer MARylation in Mmu WCE (A) and in the system reconstituted from recombinant proteins (B). (A) Mmu cell extract proteins (0.5 mg/mL) were incubated for 5 min with 100 nM DNA duplexes bearing dRP, pDEG, or flap in the presence of 5 mM MgCl2 and 0.5 mM NAD+ in the absence or presence of PARG and olaparib. (B) Recombinant proteins were incubated for 10 min with 100 nM DNA duplexes bearing dRP, pDEG, or flap in the presence of 5 mM MgCl2, 0.1 mM dNTPs, and 0.5 mM NAD+ (when indicated). Lanes 1 in A and B correspond to the initial primer (control).
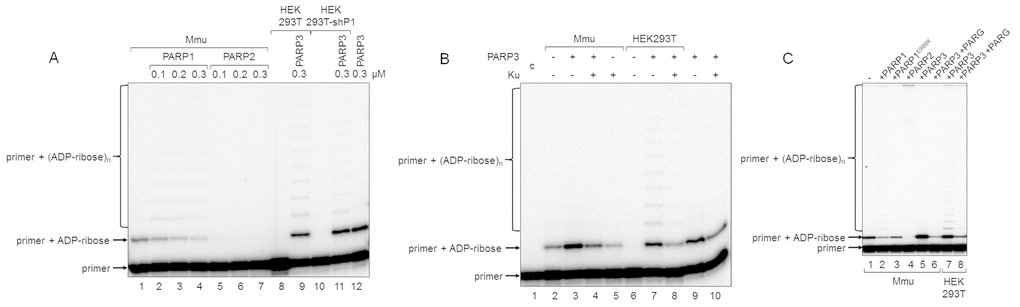
Figure 4. Influence of exogenous proteins on (ADP-ribosyl)ation of primer in WCEs. (A) WCE proteins in the absence or presence of extra recombinant proteins were incubated for 5 min with 100 nM DNA duplex dRP, 0.5 mM NAD+, and 5 mM spermine as described in the section ‘DNA (ADP-ribosyl)ation assay’. Recombinant PARP1, PARP2, or PARP3 were added to the extracts at the indicated concentrations prior to initiation of the (ADP-ribosyl)ation reaction. Lane 1, no extra recombinant proteins were added; lane 12, no extract proteins were added. (B) Mmu or HEK293T WCE proteins (0.5 mg/mL) were incubated for 5 min with 100 nM DNA duplex containing dRP moiety in the presence of 0.5 mM NAD+ and 5 mM spermine as described in the section ‘DNA (ADP-ribosyl)ation assay’. PARP3 and/or Ku (each at the final concentration of 300 nM) were added to the extracts prior to initiation of the (ADP-ribosyl)ation reaction. Lane 1 corresponds to the initial primer (control). (C) Mmu or HEK293T WCE proteins (0.5 mg/mL) were incubated at 37 °C for 5 min with 100 nM DNA duplex containing the dRP moiety in the presence of 0.5 mM NAD+ as described in the section ‘DNA (ADP-ribosyl)ation assay’. PARP1, PARP1E988K, PARP2, or PARP3 (the final concentrations of 300 nM) were initially added to the reaction mixtures and indicated. 50 nM PARG was added to some mixtures (lanes 6 and 8) after the reactions were stopped by the addition of EDTA, and the mixtures were incubated at 37 °C for another 10 min.
In this study, we used DNA substrates mimicking BER intermediates, which differ in the structure of the 5ʹ termini of downstream oligonucleotides. This fact appeared to influence the efficiency of primer MARylation (Figure 3B, compare lanes 6, 11, and 16). The efficiency of the upstream primer MARylation in the substrates decreases in a row: dRP > pDEG > Flap. DNAs bearing the 5ʹ-dRP and pDEG residues were not previously tested in the reaction of DNA (ADP-ribosyl)ation. MARylation of the upstream primer in an analogous flap-containing structure has already been demonstrated [19]. The efficiency of primer MARylation not only depends on the structure of the 5ʹ end of the downstream oligonucleotides but also differs for Mmu WCE and recombinant PARP3 (compare Figure 3A, lanes 3, 7, and 11 with Figure 3B, lanes 6, 11, and 16), which may reflect the processing of the groups at the 5ʹ termini of downstream oligonucleotides by the extract enzymes.
To determine how primer MARylation may interfere with the downstream stages of BER, we analyzed DNA synthesis in Mmu and Hgl WCEs in the presence or absence of ATP, NAD+, and olaparib (Figure S3 A and B). In the case of the reaction mixtures containing Mmu WCE and NAD+, the products that were ascribed to MARylated oligonucleotides fully or partially disappear in the presence of olaparib (Figure S3 A and B, lane 12 vs 13, lane 15 vs 16, and lane 18 vs 19) irrespectively of the presence of ATP. In all cases, the amount of the products that could be ascribed to the full-length chain insignificantly increases in the presence of ATP but their appearance is not related to DNA (ADP-ribosyl)ation.
We assumed that detectability of the MARylated primers would be dependent on the ratios of the PARP1, PARP2, PARP3, and PARG amounts, their activities in the extracts, and the relative affinities of these enzymes to damages in DNA. It is difficult to separately evaluate the activity of each PARP in the extracts and to compare the amounts of PARPs by immunological approaches due to different biological origins of the cells. However, significant information can be obtained in functional tests after the addition of certain purified proteins to the extracts.
First, we studied the kinetics of primer MARylation in the extracts using spermine as a cofactor for PARPs instead of magnesium ions to minimize primer degradation by endogenous nucleases in the WCE. The results for Mmu WCE are shown in Figure 5. In the absence of the DNA synthesis, MARylated primers can be consumed by either their further PARylation catalyzed by endogenous PARP1/PARP2 or the removal of the ADP-ribose residue by endogenous PARG. Slow time-dependent accumulation of the MARylated primer in Mmu WCE appears to reflect the balance between the activities of the above enzymes. The MARylated primers were registered in the extracts, which were characterized by low efficiency of PAR synthesis. This fact may indicate small amounts of PARP1 and PARP2 in these extracts. It should be noted that PARP1 contribution to the total PAR synthesis can reach 95% [26]. Indeed, Mmu WCE was the most inefficient in PAR synthesis compared to other extracts under study (Figure 1A). In addition, Mmu WCE demonstrates the lowest rate of PAR degradation (Figure 1B).
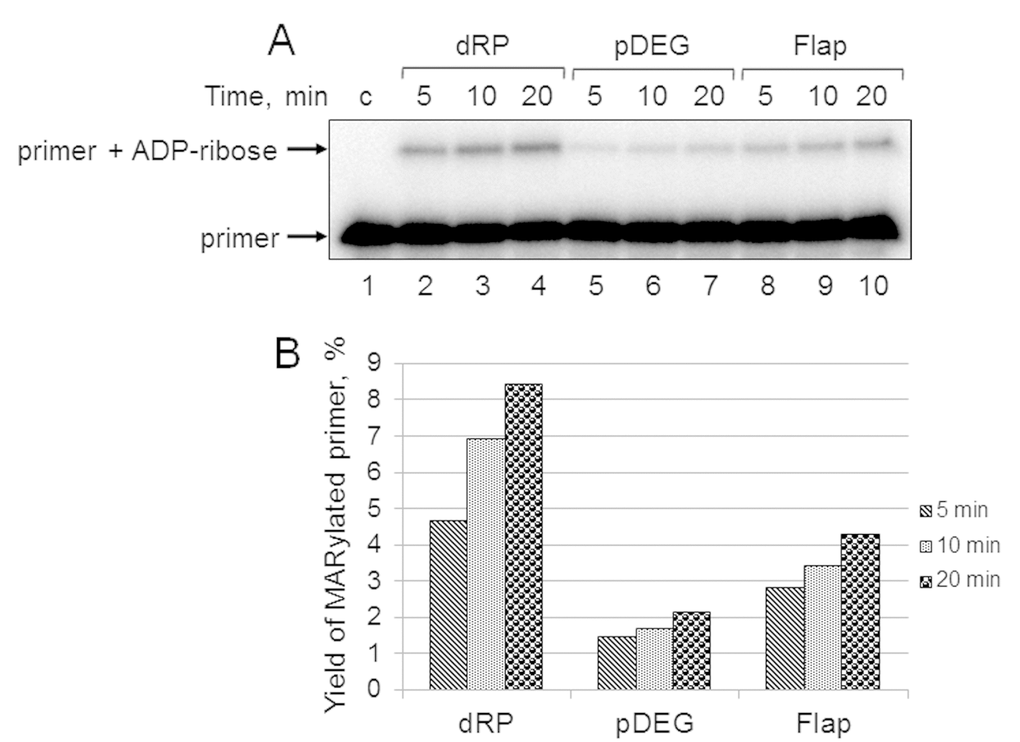
Figure 5. Kinetics of primer MARylation in Mmu WCE (A) and quantification of the reaction products (B). The Mmu cell extract proteins (0.5 mg/mL) were incubated for 5, 10, or 20 min with 100 nM DNA duplexes bearing dRP, pDEG, or flap in the presence of 0.5 mM NAD+ and 5 mM spermine as described in the section ‘DNA (ADP-ribosyl)ation assay’. Lane 1 corresponds to the initial primer (control). The yield of the MARylated primer (%) was calculated as the amount of the corresponding product normalized to overall DNA content in the lane.
In the reconstituted system, both PARP1 and PARP2 were able to PARylate the upstream primer, which was preliminary MARylated, purified, and annealed to form a nick-containing DNA duplex, and a higher efficacy was observed for PARP2 [21]. Taking into account this result, we added extra PARP1, PARP2, or PARP3 into the extracts before PAR synthesis was initiated (Figure 4A). The addition of PARP1 to Mmu WCE led to concentration-dependent disappearance of the MARylated primer, which was accompanied by the appearance of the step-by-step elongation products (compare lane 1 with lanes 2–4 in Figure 4A), while the addition of PARP2 resulted in full disappearance of the MARylated primer even at the lowest concentration of PARP2 used (compare lane 1 with lanes 5–7 in Figure 4A). In addition, primer modification products with low electrophoretic mobility, i.e., with a higher PARylation level, can be observed close to the top of the gel (Figure 4C). The addition of extra PARP1 or PARP2 to Mmu extract enhances the amount of these products (Figure 4C, compare lanes 2 and 4 with lane 1), and the amount was higher for PARP2. PARP3, when added, does not influence the amount of low electrophoretic mobility products but only increases the amount of the MARylated primer (Figure 4C, lane 5 vs lane 1). Interestingly, PARP1E988K, the mutant form of PARP1, which has only mono(ADP-ribose) transferase activity, reduces the amount of the MARylated primer (Figure 4C, lane 3 vs lane 1). Taken together, these results may testify to a very rapid and efficient PARylation of the MARylated primer by extra PARP2. These results are corroborated by a considerably higher activity of PARP2 as compared to PARP1 in PARylation of a MARylated primer in a system reconstituted from recombinant proteins [21].
Using DNAs with unmodified or purified MARylated primers, it has been shown that PARylation of a MARylated primer can be performed quite efficiently in HEK293 WCE, while the products of de novo MARylation or PARylation of the unmodified primer were practically undetectable [21]. These data are in agreement with our results for HEK293T WCE (Figure 2B). This fact may be due to either a relatively low amount of PARP3 in human cells or a competition of DNA-binding cell proteins with PARP3 for DNA. Indeed, the addition of extra PARP3 to HEK293T WCE led to the appearance of the MARylated primer and products of its PARylation (Figure 4A, lane 9 vs lane 8 and lane 11 vs lane 10). Moreover, the pattern of the products resembles that obtained with Mmu WCE with extra PARP1 (Figure 4A, lanes 2–4). A lower amount of the PARylated primer is detected in the extract of HEK293T cells, in which PARP1 production is partially inhibited by expression of a specific shRNA (HEK293T-shP1), (Figure 4A, lane 11 vs lane 9). The efficiency of conversion of the MARylated primer, which was synthesized in the WCEs of HEK293T-shP1 and HEK293T in the presence of extra PARP3, to the PARylated primer was 5.4% and 24.9%, respectively (compare lanes 9 and 11 in Figure 4A). It should be emphasized that the yield of PAR in WCEs of HEK293T-shP1 as compared to HEK293T was about 30% as determined by the test on the paper filters (data not shown).
Another cell protein that may interfere with MARylation of primers by PARP3 at the 5ʹ ends is Ku antigen (Ku), which is composed of the Ku80 and Ku70 polypeptide chains named according to their molecular masses of about 80 and 70 kDa. Ku binds double-stranded DNA ends with high affinity and is well-known for its central role as a DNA end-binding factor at the initial stage of the classical nonhomologous end-joining pathway, which is the main DNA double-strand break (DSB) repair pathway in mammalian cells [29–31]. The data on the interference of extra Ku with DNA (ADP-ribosyl)ation in the extracts are shown in Figure 4B. The addition of Ku to Mmu WCE reduced the level of primer MARylation by endogenous PARP3 by 40% (lane 5 vs lane 2). The inhibitory effect of Ku was also manifested when both PARP3 and Ku were added to the extract (compare lanes 3 and 4) or in the reaction mixtures containing purified PARP3 and Ku (compare lanes 9 and 10). In HEK293T WCE, the addition of PARP3, as expected, resulted in the appearance of the MARylated primer (compare lanes 6 and 7) and extra Ku interfered with this process (compare lanes 7 and 8).
Affinity labeling of cell extract proteins by chemically reactive DNAs mimicking BER intermediates
For a better understanding of the mechanisms and the role of DNA (ADP-ribosyl)ation, especially taking into account the above data, it is important to obtain information about the amounts in the extracts of the target proteins and other DNA binding, which may influence the binding of target proteins with DNA. Comparison of the amount of some proteins in the cell extracts may be performed by using DNA, which mimics the intermediates of the corresponding DNA repair process and contains photoactivatable groups. Synthesis of the photoreactive BER substrates can be carried out by the attachment of dCMP analogs to the 3ʹ end of a radioactively labeled primer using recombinant Polβ [32]. Here, we use exo-N-{2-[N-(4-azido-2,5-difluoro-3-chloropyridine-6-yl)-3-aminopropionyl]amino-ethyl}-2′-deoxycitidine-5′-triphosphate (FAP-dCTP) as a substrate. The chosen conditions for the synthesis of photoreactive DNA provided a full elongation of the primer by FAP-dCMP in all three BER substrates (Figure S4). The DNA synthesis was stopped by the addition of EDTA, and the reaction mixture was further used as a source of the photoreactive DNAs. It should be noted that EDTA is a mandatory component of the reaction mixtures, which prevents DNA hydrolysis s by endogenous nucleases in the extracts. The WCE or recombinant proteins were incubated with photoreactive DNAs for binding, followed by UV-light irradiation for DNA-protein cross-linking. The data on the cross-linking of proteins to the photoreactive DNAs are shown in Figure 6. The main difference between the patterns of protein cross-linking consists in extremely intensive labeling of high molecular weight proteins (70–120 kDa) in HEK293T WCE (Figure 6, lanes 4–6 vs lanes 7–9 and 10–12). Taking into account the apparent molecular masses of the observed products and our previous data concerning the photoaffinity labeling of proteins in the extracts of mammalian cells [15,33,34], we can assume that these three highly intensive bands correspond to the cross-linking products formed by PARP1, Ku80, and Ku70 (from top to bottom). The cross-linking of DNAs to individual proteins or their combinations (Figure 6, lanes 4–6 vs lanes 1–3 and lanes 13–21) led to the appearance of bands with corresponding mobility thus confirming our hypothesis. Interestingly, the intensity of the bands attributed to PARP1 cross-linking products varied depending on the type of photoreactive DNA, and the lowest intensity was observed for Pho-Flap (Figure 6, lanes 1, 2 vs lane 3; lanes 13, 14 vs lane 15). The analogous tendency is typical of HEK293T WCE (Figure 6, lanes 4, 5 vs lane 6). Only low-intensity bands, which can be attributed to the products of PARP1 cross-linking were detected in Hgl WCE, (Figure 6, lanes 7–9 vs lanes 1–3), while the corresponding products were not practically observed in 3T3 WCE (Figure 6, lanes 10–12). This observation correlates with the data shown in Figure 1A concerning an extremely high difference in the total PARylation efficiency between human and rodent cell extracts. In HEK293T WCE, the bands corresponding to the products of Ku protein cross-linking are very abundant (Figure 6, lanes 4–6), while no products of cross-linking, which could be unambiguously attributed to Ku, were observed in rodent cell extracts. Importantly, very intensive bands corresponding to the products of the Ku protein labeling by an analogous photoreactive DNA were observed in HeLa WCE [34] but not in the mouse embryonic fibroblast extract [15]. This is fully consistent with previously published data on a higher abundance of Ku in cells of primates compared to other mammals [35].
![Interaction of proteins with different types of photoreactive DNA. Photoaffinity modification was performed as described in the section ‘Photoaffinity modification of proteins’ using 100 nM DNAs and 1 mg/mL cell extract proteins (HEK293T, lanes 4–6; Hgl, lanes 7–9; 3T3, lanes 10–12), as well as purified PARP1 (100 nM, lanes 1–3 and 13–15), PARP1 + Ku (100 nM each, lanes 16–18), Ku (100 nM, lanes 19–21), Polβ (200 nM, lanes 25–27), FEN1 (100 nM, lanes 28–30), and HMGB1 (300 nM, lanes 31–33). Lanes 22–24 (control) correspond to the UV-light irradiated aliquots of the reaction mixtures for photoreactive DNA synthesis, which contained 100 nM Polβ. The proteins were separated by 12.5% SDS-PAGE and the proteins cross-linked to [32P]-labeled DNAs were visualized by autoradiography. The structures of the photoreactive DNAs are schematically shown at the top. The asterisk denotes the FAP-dCMP residue.](/article/101959/figure/f6/large)
Figure 6. Interaction of proteins with different types of photoreactive DNA. Photoaffinity modification was performed as described in the section ‘Photoaffinity modification of proteins’ using 100 nM DNAs and 1 mg/mL cell extract proteins (HEK293T, lanes 4–6; Hgl, lanes 7–9; 3T3, lanes 10–12), as well as purified PARP1 (100 nM, lanes 1–3 and 13–15), PARP1 + Ku (100 nM each, lanes 16–18), Ku (100 nM, lanes 19–21), Polβ (200 nM, lanes 25–27), FEN1 (100 nM, lanes 28–30), and HMGB1 (300 nM, lanes 31–33). Lanes 22–24 (control) correspond to the UV-light irradiated aliquots of the reaction mixtures for photoreactive DNA synthesis, which contained 100 nM Polβ. The proteins were separated by 12.5% SDS-PAGE and the proteins cross-linked to [32P]-labeled DNAs were visualized by autoradiography. The structures of the photoreactive DNAs are schematically shown at the top. The asterisk denotes the FAP-dCMP residue.
The intensity of the bands corresponding to the cross-linking products with molecular mass of about 50 kDa is comparable for all extracts, and these products may be easily attributed to recombinant Polβ since the preparations of photoreactive DNA already contain this protein, which was used for the incorporation of FAP-dCMP (Figure 6, lanes 22–24). Therefore, the product corresponding to Polβ is present in all samples and can serve as an internal control. It should be noted that 200 nM Polβ (lanes 25–27) produced stronger signal than endogenous Polβ of the extracts. The intensive products with a higher apparent molecular mass than that of Polβ are observed in lanes 6, 9, and 12 corresponding to the Pho-Flap DNA. These products can be attributed to FEN1. Purified recombinant FEN1 (lanes 28–30) generates the products with the expected electrophoretic mobility.
Another DNA-binding protein, which may be involved in the BER process, is HMGB1. HMGB1 is a nuclear nonhistone DNA-binding protein, which belongs to the high-mobility group box family of proteins [36]. We have shown earlier that HMGB1 is a BER cofactor capable of modulating the BER capacity in cells [37]. Hgl and 3T3 WCEs demonstrate comparable levels of the products, which can be attributed to HMGB1 (lanes 7–12) according to their electrophoretic mobility corresponding to the mobility of the products formed by purified HMGB1 (lanes 31–33). In the case of HEK293T WCE, the corresponding bands have higher intensities (lanes 4–6 vs lanes 7–12). In all cases, the Pho-Flap DNA more efficiently cross-links to HMGB1 than other photoreactive DNAs (compare lanes 6, 9, 12, and 33 with lanes 4, 5, 6, 7, 8, 10, 11, 31, and 32). This pattern of cross-linking is in line with the ability of HMGB1 to bind more efficiently flap-containing DNAs than other BER substrates as revealed by EMSA [37].
Using Pho-dRP DNA, we performed photoaffinity modification of all three recombinant PARPs presented alone or in different combinations and also 3T3, Hgl, and HEK293T cell extracts (Figure S5). PARP3 demonstrated the considerably lower level of cross-linking as compared to PARP1 and PARP2 (lane 3 vs lanes 1 and 2) at the equal concentrations of DNA and proteins. No products, which could be ascribed to cross-links of PARP2 with DNA according to electrophoretic mobility, were detected in the extracts (compare lane 8 with lanes 9–11); this fact may indicate a small number of PARP2 copies. The nature of DNA-protein covalent adducts in HEK293T and Hgl WCEs, which we attribute to PARP1, was additionally confirmed by cross-linking in the presence of NAD+ (Figure S5B). The amount of the PARP1-dependent protein–DNA complex was reduced and slower migrating material was now observed both with purified PARP1 (compare lanes 1–3 and lanes 13–15 with lanes 4–6 and lanes 16–18 in Figure 5S B) and in WCEs (compare lanes 7–9 with lanes 10–12 and lanes 19–21 with lanes 22–24 in Figure 5S B). This fact is consistent with cross-linking of DNA probes with PARylated PARP1. The smeared slower migrating material appeared due to variable length of the attached PAR. This phenomenon has already been demonstrated by us earlier [6,15].