LINC00963 facilitates acute myeloid leukemia development by modulating miR-608/MMP-15
Abstract
Despite continuous improvements of AML therapy, the prognosis of AML patients remains unsatisfactory. Recently, lncRNAs have been reported to participate in the development of AML. Our data demonstrated that MMP15 and LINC00963 were upregulated and miR-608 was decreased in AML cells (THP-1, HL-60, HEL and MOLM-13) compared to HS-5 cells. RT-qPCR results showed that LINC00963 levels were higher in the serum and bone marrow of AML cases than in controls. Moreover, overexpression of LINC00963 promoted AML cell growth and EMT progression in both THP-1 and HL-60 cells. Furthermore, miR-608 levels were downregulated in the serum and bone marrow of AML cases compared with controls, and Pearson’s correlation analysis indicated that LINC00963 was negatively correlated with miR-608 in the serum and bone marrow of AML samples. In addition, we demonstrated that LINC00963 sponged miR-608 expression and that MMP-15 was a target of miR-608 in AML cells. Finally, rescue experiments indicated that ectopic expression of LINC00963 accelerated cell growth and EMT development by modulating MMP-15. These data demonstrated that LINC00963 acted as an oncogene and may be a potential target for AML treatment.
Introduction
Acute myeloid leukemia (AML) remains the most prevalent hematological tumor [1–3]. AML is characterized by impaired interference and apoptosis, uncontrolled proliferation and differentiation in leukemic cells [4–6]. Several risk factors, including age, white blood cell count, cytogenetic risk and gene mutations, have been reported to be related to AML development [5, 7–9]. Despite progress in the treatment of AML, the overall survival rate is still unsatisfactory [10–12]. Thus, there is a need to identify novel molecular mechanisms and treatment targets for AML.
LncRNAs are widely found in mammals and are a group of noncoding RNA transcripts longer than 200 nt with little or no protein-coding ability [13–18]. Growing studies have illustrated that lncRNAs can act as oncogenes or cancer suppressor genes in most tumors, including osteosarcoma, glioblastoma, gastric tumor, gallbladder carcinoma and AML [19–24]. Studies have suggested that lncRNAs play vital roles in a variety of cell activities, such as cell metabolism, invasion, differentiation and migration [25–30]. Recently, LINC00963, a newly discovered lncRNA, has been reported to be involved in the development of several tumors, such as breast cancer, ovarian tumors, esophageal tumors, cutaneous carcinoma, melanoma, hepatocellular carcinoma and prostate cancer [31–37]. However, the clinical relevance and cell function of LINC00963 in AML are still poorly characterized.
We first measured LINC00963 in AML cells, and RT-qPCR analysis demonstrated that LINC00963 was upregulated in AML cells (THP-1, HL-60, HEL and MOLM-13) compared to HS-5 cells. RT-qPCR analysis showed that LINC00963 levels were higher in the serum and bone marrow of AML cases than in controls. Furthermore, overexpression of LINC00963 promoted AML cell growth and EMT progression in both THP-1 and HL-60 cells.
Results
MMP15, LINC00963 and miR-608 levels in AML cells
Frist, RT-qPCR analysis was utilized to determine MMP15, LINC00963 and miR-608 levels in AML cells. MMP15 was overexpressed in AML cells (THP-1, HL-60, HEL and MOLM-13) compared with HS-5 cells (Figure 1A). LINC00963 was upregulated in AML cells (THP-1, HL-60, HEL and MOLM-13) compared to HS-5 cells (Figure 1B). Moreover, miR-608 was decreased in AML cells (THP-1, HL-60, HEL and MOLM-13) compared to HS-5 cells (Figure 1C).
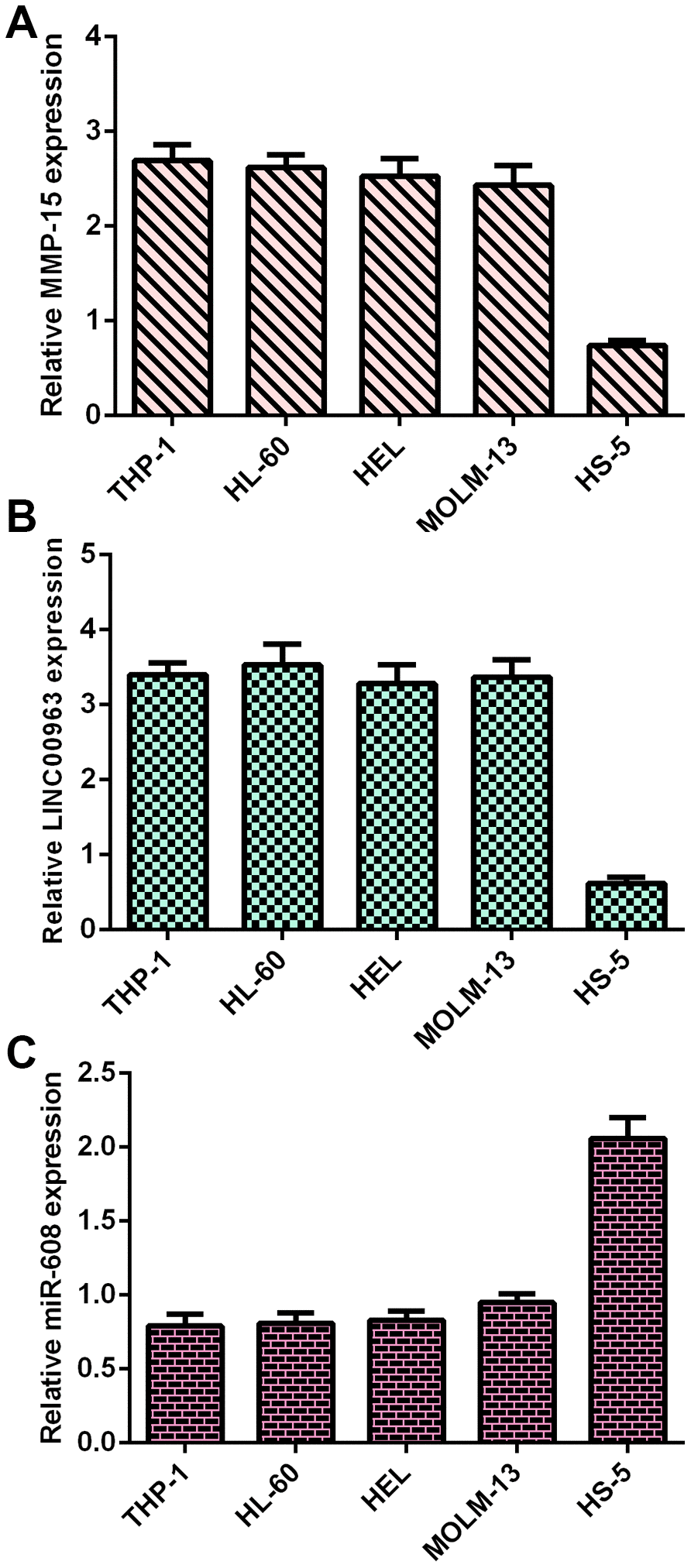
Figure 1. MMP15, LINC00963 and miR-608 levels in AML cells. (A) MMP15 was overexpressed in AML cells (THP-1, HL-60, HEL and MOLM-13) compared with HS-5 cells. GAPDH was used as the internal control. (B) The expression of LINC00963 was determined by RT-qPCR analysis. GAPDH was used as the internal control. (C) miR-608 was decreased in AML cells (THP-1, HL-60, HEL and MOLM-13) compared to HS-5 cells. U6 was used as the internal control for miR-608.
LINC00963 level in AML specimens
Then, we measured LINC00963 in the serum and bone marrow of AML cases and controls. The data illustrated that LINC00963 levels were higher in the bone marrow of AML cases than in controls (Figure 2A). Moreover, LINC00963 levels were upregulated in the serum of AML cases compared with controls (Figure 2B).
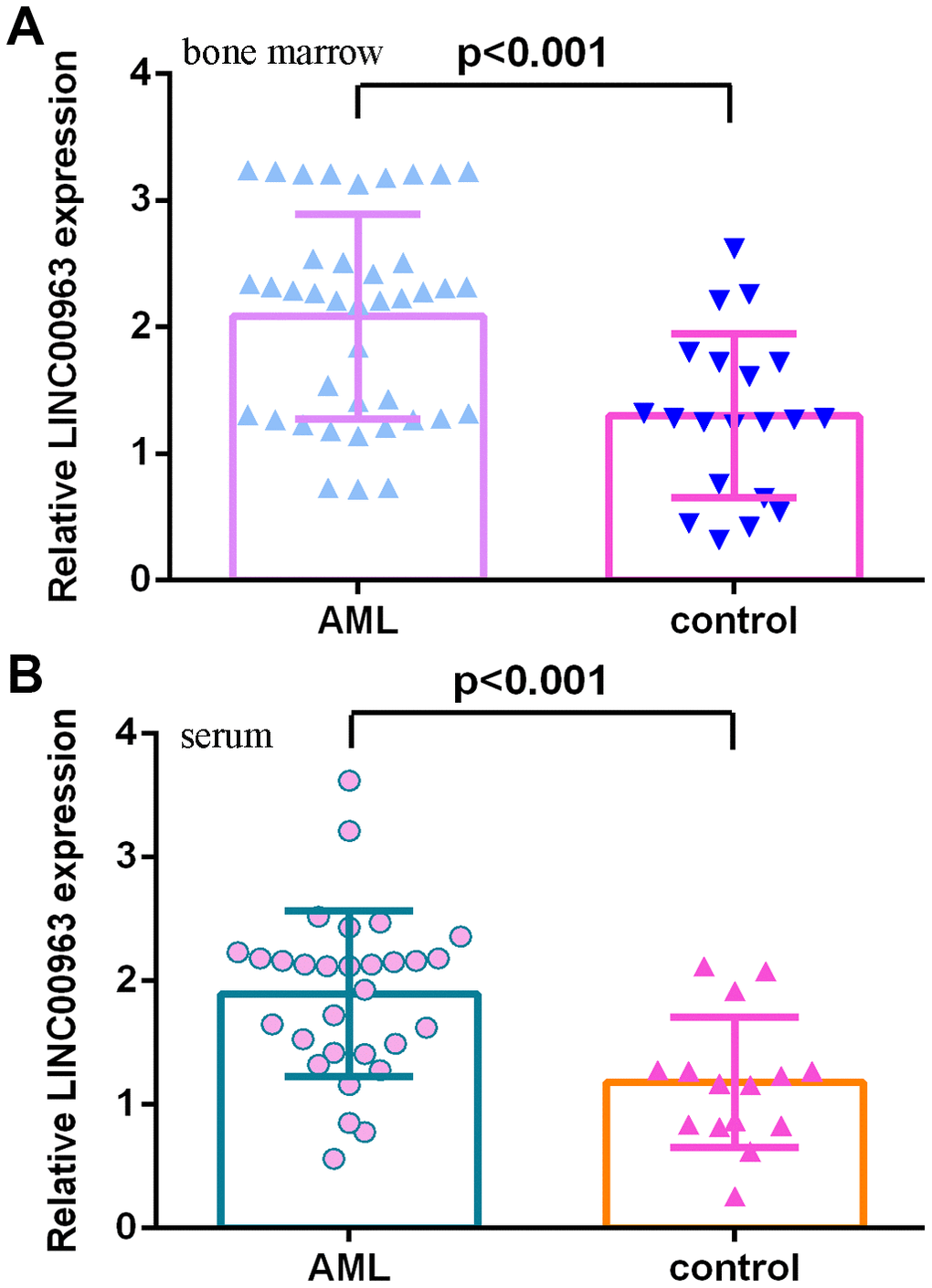
Figure 2. LINC00963 level in AML specimens. (A) LINC00963 levels were higher in the bone marrow of AML cases than in controls. (B) The expression of LINC00963 in the serum of AML cases and controls was measured by RT-qPCR. GAPDH was used as the internal control.
miR-608 level in AML specimens
Furthermore, we measured miR-608 in the serum and bone marrow of AML cases and controls. The results demonstrated that miR-608 levels were lower in the bone marrow of AML cases than in controls (Figure 3A). Pearson’s correlation analysis indicated that LINC00963 was negatively correlated with miR-608 in the bone marrow of AML samples (Figure 3B). Moreover, miR-608 levels were downregulated in the serum of AML cases compared with controls (Figure 3C). Pearson’s correlation analysis showed that LINC00963 was negatively correlated with miR-608 in the serum of AML samples (Figure 3D).
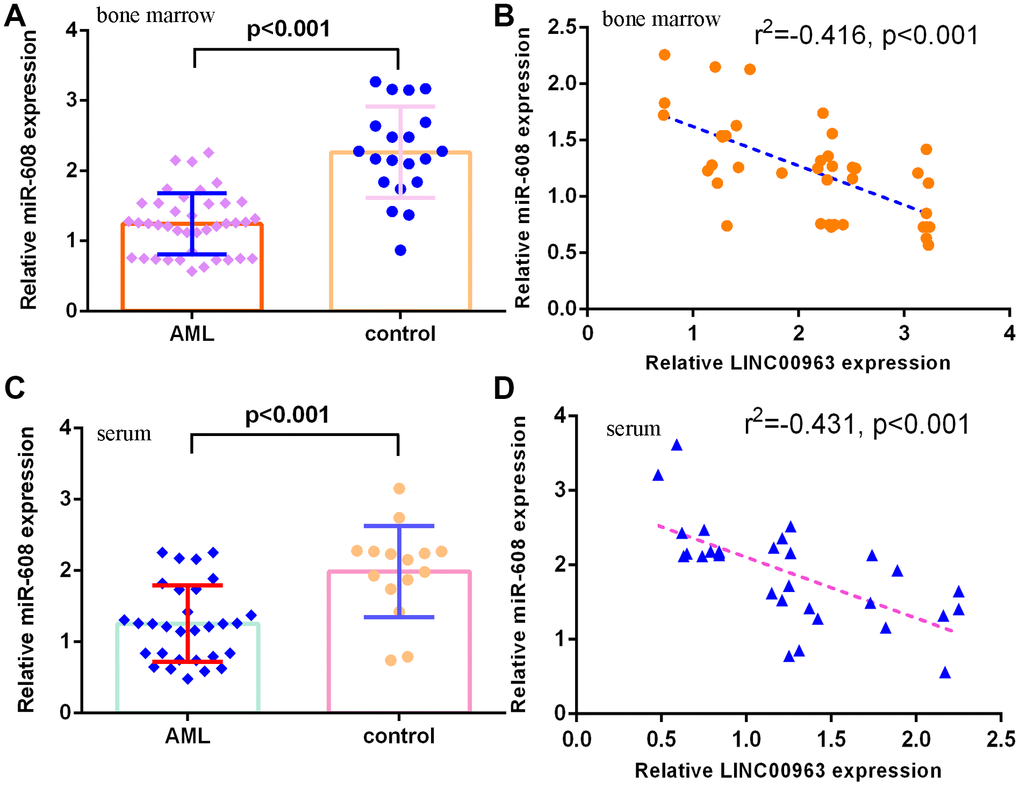
Figure 3. miR-608 levels in AML specimens. (A) The expression of miR-608 in the bone marrow of AML cases and controls was measured by RT-qPCR. (B) Pearson’s correlation analysis indicated that LINC00963 was negatively correlated with miR-608 in the bone marrow of AML samples. (C) miR-608 levels were downregulated in the serum of AML cases compared with controls. (D) Pearson’s correlation analysis sowed that LINC00963 was negatively correlated with miR-608 in the serum of AML samples. U6 was used as the internal control.
Overexpression of LINC00963 promoted AML cell growth and EMT progression
The level of LINC00963 was significantly upregulated in both AML cell lines THP-1 and HL-60 after treatment with pcDNA-LINC00963 (Figure 4A). The CCK-8 assay indicated that overexpression of LINC00963 accelerated cell growth in both THP-1 and HL-60 cells (Figure 4B). Elevated expression of LINC00963 upregulated ki-67 expression in both THP-1 and HL-60 cells (Figure 4C). RT-qPCR assays illustrated that elevated expression of LINC00963 increased PCNA levels in both THP-1 and HL-60 cells (Figure 4D). Moreover, ectopic expression of LINC00963 enhanced Vimentin, N-cadherin and ZEB1 expression and decreased E-cadherin expression in both THP-1 and HL-60 cells (Figure 4E).
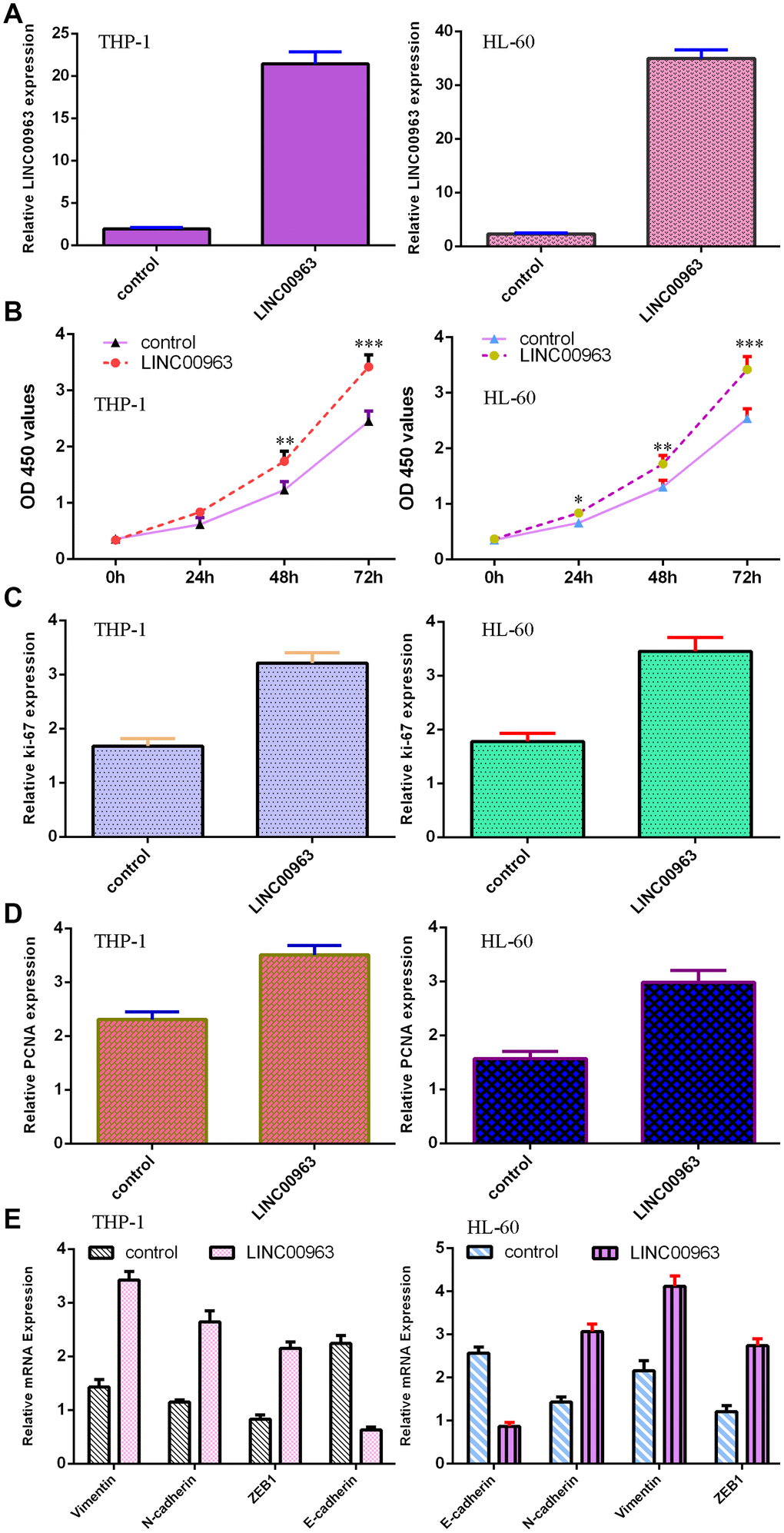
Figure 4. Overexpression of LINC00963 promoted AML cell growth and EMT progression. (A) The expression of LINC00963 was measured by RT-qPCR in both THP-1 and HL-60 AML cells. (B) CCK-8 assay showed that overexpression of LINC00963 accelerated cell growth in both THP-1 and HL-60 cells. (C) Elevated expression of LINC00963 facilitated ki-67 expression in both THP-1 and HL-60 cells. (D) RT-qPCR assay indicated that elevated expression of LINC00963 accelerated PCNA levels in both THP-1 and HL-60 cells. (E) Ectopic expression of LINC00963 enhanced Vimentin, N-cadherin and ZEB1 expression and decreased E-cadherin expression in both THP-1 and HL-60 cells. *p<0.05, **p<0.01 and ***p<0.001. GAPDH was used as the internal control.
LINC00963 inhibited miR-608 expression in AML cells
An online database (starBase) predicted that miR-608 was a potential target of LINC00963 (Figure 5A). The level of miR-608 was significantly upregulated in THP-1 cells after treatment with the miR-608 mimic (Figure 5B). Luciferase reporter assays verified that elevated expression of miR-608 decreased the luciferase value in the LINC00963-wt group but did not change the luciferase value in the LINC00963-mut group (Figure 5C). Overexpression of LINC00963 suppressed miR-608 levels in THP-1 cells (Figure 5D).
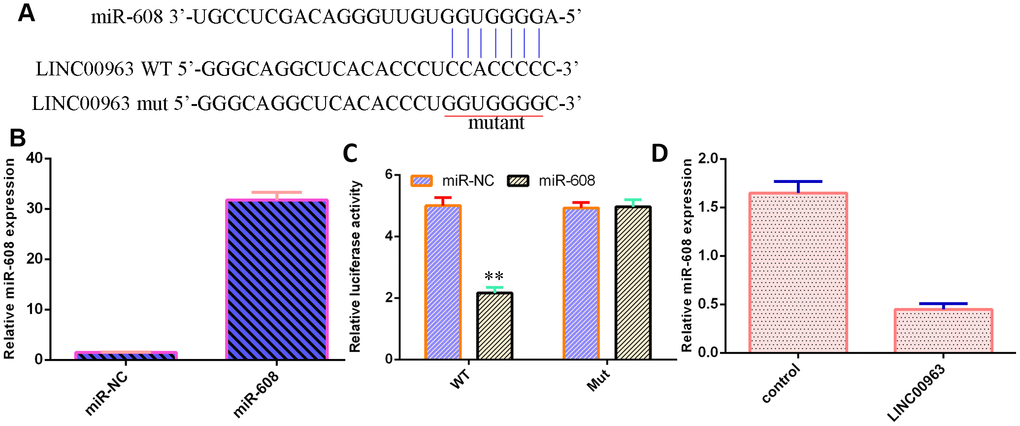
Figure 5. LINC00963 inhibited miR-608 expression in AML cells. (A) An online database (starBase) predicted that miR-608 was a potential target of LINC00963. (B) The level of miR-608 was determined by RT-qPCR. (C) Luciferase reporter assays verified that elevated expression of miR-608 decreased the luciferase value in the LINC00963-wt group but did not change the luciferase value in the LINC00963-mut group. (D) Overexpression of LINC00963 suppressed miR-608 levels in THP-1 cells. **p<0.01. U6 was used as the internal control.
MMP-15 was a target of miR-608
An online database (TargetScan) predicted that MMP-15 was a potential target of miR-608 (Figure 6A). Luciferase reporter analysis verified that elevated expression of miR-608 decreased the luciferase value in the MMP-15-wt group but did not change the luciferase value in the MMP-15-mut group (Figure 6B). Overexpression of miR-608 suppressed MMP-15 levels in THP-1 cells (Figure 6C). Ectopic expression of LINC00963 upregulated MMP-15 levels in THP-1 cells (Figure 6D).
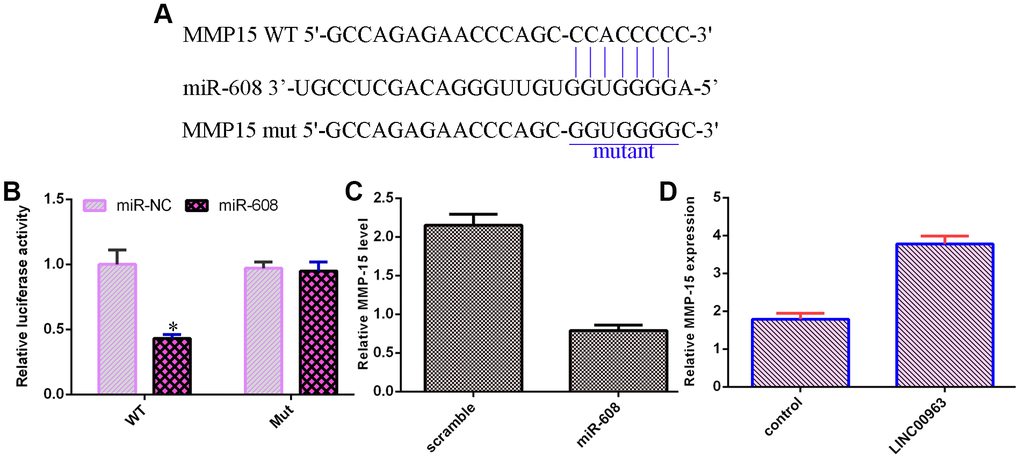
Figure 6. MMP-15 was a target of miR-608. (A) An online database (TargetScan) predicted that MMP-15 was a potential target of miR-608. (B) Luciferase reporter assays verified that elevated expression of miR-608 decreased the luciferase value in the MMP-15-wt group but did not change the luciferase value in the MMP-15-mut group. (C) Overexpression of miR-608 suppressed MMP-15 levels in THP-1 cells. (D) Ectopic expression of LINC00963 upregulated MMP-15 levels in THP-1 cells. *p<0.05. GAPDH was used as the internal control.
Ectopic expression of LINC00963 increased cell growth and EMT development by modulating MMP-15
To further study the function of LINC00963 and MMP-15 in AML development, we treated LINC00963-overexpressing THP-1 cells with an siRNA-MMP-15 plasmid. MMP-15 expression was downregulated in THP-1 cells after treatment with the siRNA-MMP-15 plasmid, as demonstrated by RT-qPCR assay (Figure 7A). MMP-15 knockdown inhibited cell growth in LINC00963-overexpressing THP-1 cells (Figure 7B). Knockdown of MMP-15 suppressed ki-67 (Figure 7C) and PCNA levels (Figure 7D) in LINC00963-overexpressing THP-1 cells. Furthermore, downregulation of MMP-15 suppressed Vimentin, N-cadherin and ZEB1 levels and upregulated E-cadherin expression in LINC00963-overexpressing THP-1 cells (Figure 7E).
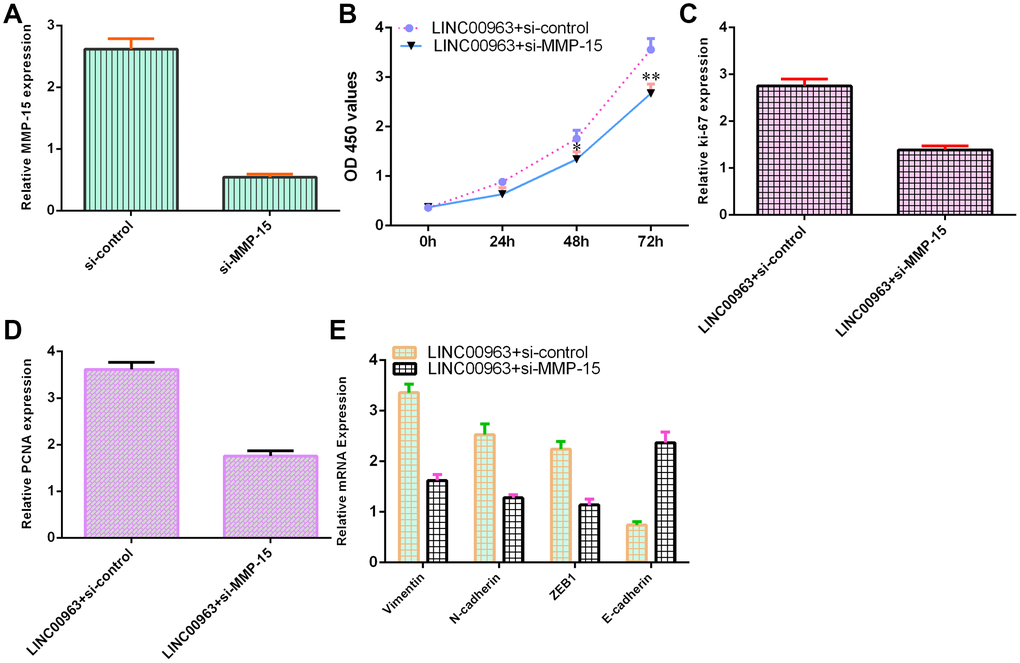
Figure 7. Ectopic expression of LINC00963 increased cell growth and EMT development by modulating MMP-15. (A) The level of MMP-15 was determined by RT-qPCR. (B) CCK-8 assay was performed to detect cell proliferation. (C) The level of ki-67 was measured by RT-qPCR. (D) The expression of PCNA was measured by RT-qPCR. (E) Downregulation of MMP-15 suppressed Vimentin, N-cadherin and ZEB1 levels and upregulated E-cadherin expression in LINC00963-overexpressing THP-1 cells. *p<0.05 and**p<0.01. GAPDH was used as the internal control.
Discussion
Despite continuous advances in AML therapy, the prognosis of AML patients remains unsatisfactory [38–40]. Recently, lncRNAs have been reported to participate in the development of AML [5, 19, 41–43]. This research explored the role of LINC00963 in the progression of AML. Our data showed that MMP15 and LINC00963 were upregulated and miR-608 was decreased in AML cells (THP-1, HL-60, HEL and MOLM-13) compared to HS-5 cells. RT-qPCR results demonstrated that LINC00963 levels were higher in the serum and bone marrow of AML cases than in controls. Moreover, overexpression of LINC00963 promoted AML cell growth and EMT progression in both THP-1 and HL-60 cells. Moreover, miR-608 levels were downregulated in the serum and bone marrow of AML cases compared with controls, and Pearson’s correlation analysis indicated that LINC00963 was negatively correlated with miR-608 in the serum and bone marrow of AML samples. In addition, we determined that LINC00963 sponged miR-608 expression and that MMP-15 was a target of miR-608 in AML cells. Finally, rescue experiments indicated that ectopic expression of LINC00963 accelerated cell growth and EMT development by modulating MMP-15. These data illustrated that LINC00963 acted as an oncogene and may be a potential target for AML cases.
Recently, LINC00963, a novel lncRNA, has been reported to be involved in the development of several tumors, such as breast cancer, ovarian tumors, esophageal tumors, cutaneous carcinoma, melanoma, hepatocellular carcinoma and prostate cancer [31–37]. Wu et al [44]. noted that LINC00963 was upregulated in breast tumor specimens, and higher levels of LINC00963 were associated with TNM stage, differentiation grade and lymph node metastasis. Knockdown of LINC00963 suppressed cell invasion and proliferation and accelerated apoptosis, partly regulating miR-625/HMGA1. Liu et al [36]. demonstrated that LINC00963 induced ovarian tumor EMT progression and growth by regulating CHI3L1/miR-378g. Liu et al [35]. noted that LINC00963 enhanced esophageal tumor invasion by modulating RAB14/miR-214-5p. Zhou et al [45]. indicated that LINC00963 accelerated osteosarcoma cell invasion and growth by inhibiting miR-204-3p/FN1. However, the clinical relevance and cell function of LINC00963 in AML are still poorly characterized. We first measured LINC00963 in AML cells, and RT-qPCR analysis illustrated that LINC00963 was upregulated in AML cells (THP-1, HL-60, HEL and MOLM-13) compared to HS-5 cells. Then, we determined LINC00963 levels in the serum and bone marrow of AML cases and controls, and RT-qPCR analysis showed that LINC00963 levels were higher in the serum and bone marrow of AML cases than in controls. Furthermore, overexpression of LINC00963 promoted AML cell growth and EMT progression in both THP-1 and HL-60 cells.
A growing number of lncRNAs have emerged as ceRNAs that protect mRNAs from targeting by miRNAs [46, 47]. A previous study showed that LOC285758 induced AML cell invasion by inhibiting miR-204-5p [48]. Tian et al [5]. noted that lncRNA SBF2-AS1 regulated AML cell growth by regulating miR-188-5p. Gan et al [49]. showed that lncRNA ZFAS1 knockdown decreased AML development by modulating the miR-150/Myb/Sp1 pathway. Dong et al [50]. indicated that HOXA-AS2 knockdown inhibited AML chemoresistance by sponging miR-520c-3p/S100A4. Moreover, Jiao et al [33]. demonstrated that overexpression of LINC00963 facilitated melanoma development by sponging miR-608/NACC1. An online database (starBase) predicted that miR-608 was a potential target of LINC00963. Luciferase reporter assays verified that elevated expression of miR-608 decreased the luciferase value in the LINC00963-wt group but did not change the luciferase value in the LINC00963-mut group. These results indicated that LINC00963 inhibited miR-608 expression in AML cells. Furthermore, we identified MMP-15 as a target gene of miR-608 in AML cells. Wu et al. showed that MMP-15 was overexpressed in AML cells and in peripheral blood and bone marrow of AML. Our study demonstrated that ectopic expression of LINC00963 accelerated cell growth and EMT development by modulating MMP-15.
Our results illustrated that LINC00963 was upregulated in AML cells and that LINC00963 levels were higher in the serum and bone marrow of AML cases than in controls. Ectopic expression of LINC00963 accelerated cell growth and EMT development by modulating the miR-608/MMP-15 axis.
Materials and Methods
Specimens, cell culture and transfection
Serum and bone marrow samples were obtained from AML patients and control cases at the Affiliated Cancer Hospital of Zhengzhou University. Our research was approved by the Clinical Ethics Committees Board of the Affiliated Cancer Hospital of Zhengzhou University, and written informed consent was obtained from each participant. HS-5 and AML cells (THP-1, HL-60, HEL and MOLM-13) were obtained from ATCC and cultured in RPMI-1640 medium supplemented with antibiotics and FBS. miR-608 mimic, siRNA-MMP-15, pcDNA-LINC00963 and controls were purchased from Genema and transfected using LipofectamineTM2000.
Cell proliferation assays
Cell growth was determined with a CCK-8 assay (Dojindo, Japan). Cells were cultured in 96-well plates and measured at 0, 1, 2 and 3 days. The cell number was determined from the optical density (OD) at 450 nm.
RT-qPCR
Total RNA from all specimens and cells was isolated with a TRIzol kit (Ambion, USA). For mRNA, miRNA and lncRNA expression, RT-qPCR was used on an ABI7500 PCR system (Applied Biosystems, USA) using a SYBR kit (TaKaRa, China). The fold change of these genes was determined with the 2-ΔΔCt method. U6 and GAPDH were used as controls. The primer sequences were as follows: LINC00963, 5’-GGTAA ATCGA GGCCC AGAGAT-3’ (F), 5’-ACGTG GATGA CAGCG TGTGA-3’ (R); GAPDH, 5’-CATGA GAAGT ATGAC AACAG CCT-3’ (F), 5’- AGTCC TTCCA CGATAC CAAAGT-3’ (R); miR-608 5’-AGGGG TGGTG TTGGG ACAGC-3’; and U6, 5’-GCGCG TCGTG AAGCG TTC-3’ (F), 5’- GTGCA GGGTC CGAGG -3’ (R).
Luciferase reporter assay
For the luciferase reporter assay, wild-type LINC00963 and wild-type MMP-15 (LINC00963-wt and MMP-15-wt) or sequences with mutant binding sites (LINC00963-mut and MMP-15-mut) were cloned downstream of the Renilla luciferase gene in the pRLTK vector (Promega, USA). Each vector was cotransfected into cells with miR-608 mimic or control (RiboBio, China). Luciferase analysis was carried out with a dual luciferase detection reagent (Promega, USA), and the relative luciferase value was calculated from Renilla luciferase activity.
Statistical analysis
The data values are presented as the mean ± SD. Analysis of statistical differences was assessed with Student’s t-test using SPSS 18.0 (Chicago, USA). The significance level was set at P<0.05. Spearman’s correlation analysis was performed to study the correlation.
Author Contributions
Concept and Supervision - Wenli Zuo, Keshu Zhou, Mei Deng; Study Design - Jian Zhou, Yongping Song; Resource and Materials Yongping Song; Data Collection and/or Processing - Wenli Zuo, Keshu Zhou,Yongping Song; Analysis and/or Interpretation - Wenli Zuo, Keshu Zhou,Yongping Song; Literature Search - Quande Lin, Qingsong Yin, Chunlei Zhang; Writing - Wenli Zuo, Keshu Zhou, Mei Deng; Critical Reviews - Wenli Zuo, Chunlei Zhang, Jian Zhou, Yongping Song.
Conflicts of Interest
All the authors in this paper declared that we have no conflicts of interest to this work.
Funding
This study was supported by the National Natural Science Foundation of China (Grant 81470336).
Editorial Note
&
This corresponding author has a verified history of publications using a personal email address for correspondence.
References
-
1.
Foran JM. New prognostic markers in acute myeloid leukemia: perspective from the clinic. Hematology Am Soc Hematol Educ Program. 2010; 2010:47–55. https://doi.org/10.1182/asheducation-2010.1.47 [PubMed]
-
2.
Fayyad-Kazan H, Bitar N, Najar M, Lewalle P, Fayyad-Kazan M, Badran R, Hamade E, Daher A, Hussein N, ElDirani R, Berri F, Vanhamme L, Burny A, et al. Circulating miR-150 and miR-342 in plasma are novel potential biomarkers for acute myeloid leukemia. J Transl Med. 2013; 11:31. https://doi.org/10.1186/1479-5876-11-31 [PubMed]
-
3.
Zhu B, Xi X, Liu Q, Cheng Y, Yang H. MiR-9 functions as a tumor suppressor in acute myeloid leukemia by targeting CX chemokine receptor 4. Am J Transl Res. 2019; 11:3384–97. [PubMed]
-
4.
Peters AH, Schwaller J. Epigenetic mechanisms in acute myeloid leukemia. Prog Drug Res. 2011; 67:197–219. https://doi.org/10.1007/978-3-7643-8989-5_10 [PubMed]
-
5.
Tian YJ, Wang YH, Xiao AJ, Li PL, Guo J, Wang TJ, Zhao DJ. Long noncoding RNA SBF2-AS1 act as a ceRNA to modulate cell proliferation via binding with miR-188-5p in acute myeloid leukemia. Artif Cells Nanomed Biotechnol. 2019; 47:1730–37. https://doi.org/10.1080/21691401.2019.1608221 [PubMed]
-
6.
Yi YY, Yi J, Zhu X, Zhang J, Zhou J, Tang X, Lin J, Wang P, Deng ZQ. Circular RNA of vimentin expression as a valuable predictor for acute myeloid leukemia development and prognosis. J Cell Physiol. 2019; 234:3711–19. https://doi.org/10.1002/jcp.27145 [PubMed]
-
7.
Mayer K, Hahn-Ast C, Schwab K, Schmidt-Wolf IG, Brossart P, Glasmacher A, von Lilienfeld-Toal M. Long-term follow-up of cladribine, high-dose cytarabine, and idarubicin as salvage treatment for relapsed acute myeloid leukemia and literature review. Eur J Haematol. 2020; 104:538–45. https://doi.org/10.1111/ejh.13395 [PubMed]
-
8.
Chen XX, Lin J, Qian J, Qian W, Yang J, Ma JC, Deng ZQ, Xie D, An C, Tang CY, Qian Z. Dysregulation of miR-124-1 predicts favorable prognosis in acute myeloid leukemia. Clin Biochem. 2014; 47:63–66. https://doi.org/10.1016/j.clinbiochem.2013.09.020 [PubMed]
-
9.
Vázquez I, Maicas M, Marcotegui N, Conchillo A, Guruceaga E, Roman-Gomez J, Calasanz MJ, Agirre X, Prosper F, Odero MD. Silencing of hsa-miR-124 by EVI1 in cell lines and patients with acute myeloid leukemia. Proc Natl Acad Sci USA. 2010; 107:E167–68. https://doi.org/10.1073/pnas.1011540107 [PubMed]
-
10.
Gurnari C, Voso MT, Maciejewski JP, Visconte V. From bench to bedside and beyond: therapeutic scenario in acute myeloid leukemia. Cancers (Basel). 2020; 12:357. https://doi.org/10.3390/cancers12020357 [PubMed]
-
11.
Gao XN, Lin J, Li YH, Gao L, Wang XR, Wang W, Kang HY, Yan GT, Wang LL, Yu L. MicroRNA-193a represses C-kit expression and functions as a methylation-silenced tumor suppressor in acute myeloid leukemia. Oncogene. 2011; 30:3416–28. https://doi.org/10.1038/onc.2011.62 [PubMed]
-
12.
Schwind S, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Holland KB, Margeson D, Becker H, Whitman SP, Wu YZ, Metzeler KH, Powell BL, Kolitz JE, et al. BAALC and ERG expression levels are associated with outcome and distinct gene and microRNA expression profiles in older patients with de novo cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. Blood. 2010; 116:5660–69. https://doi.org/10.1182/blood-2010-06-290536 [PubMed]
-
13.
Li Z, Li X, Chen X, Li S, Ho IH, Liu X, Chan MT, Wu WK. Emerging roles of long non-coding RNAs in neuropathic pain. Cell Prolif. 2019; 52:e12528. https://doi.org/10.1111/cpr.12528 [PubMed]
-
14.
Chen C, Tan H, Bi J, Li L, Rong T, Lin Y, Sun P, Liang J, Jiao Y, Li Z, Sun L, Shen J. LncRNA-SULT1C2A regulates Foxo4 in congenital scoliosis by targeting rno-miR-466c-5p through PI3K-ATK signalling. J Cell Mol Med. 2019; 23:4582–91. https://doi.org/10.1111/jcmm.14355 [PubMed]
-
15.
Zou Y, Zhong Y, Wu J, Xiao H, Zhang X, Liao X, Li J, Mao X, Liu Y, Zhang F. Long non-coding PANDAR as a novel biomarker in human cancer: a systematic review. Cell Prolif. 2018; 51:e12422. https://doi.org/10.1111/cpr.12422 [PubMed]
-
16.
Zhu S, Fu W, Zhang L, Fu K, Hu J, Jia W, Liu G. LINC00473 antagonizes the tumour suppressor miR-195 to mediate the pathogenesis of Wilms tumour via IKKα. Cell Prolif. 2018; 51:e12416. https://doi.org/10.1111/cpr.12416 [PubMed]
-
17.
Zhao J, Zhang C, Gao Z, Wu H, Gu R, Jiang R. Long non-coding RNA ASBEL promotes osteosarcoma cell proliferation, migration, and invasion by regulating microRNA-21. J Cell Biochem. 2018; 119:6461–69. https://doi.org/10.1002/jcb.26671 [PubMed]
-
18.
Zhang J, Yin M, Peng G, Zhao Y. CRNDE: an important oncogenic long non-coding RNA in human cancers. Cell Prolif. 2018; 51:e12440. https://doi.org/10.1111/cpr.12440 [PubMed]
-
19.
Zheng J, Song Y, Li Z, Tang A, Fei Y, He W. The implication of lncRNA expression pattern and potential function of lncRNA RP4-576H24.2 in acute myeloid leukemia. Cancer Med. 2019; 8:7143–60. https://doi.org/10.1002/cam4.2518 [PubMed]
-
20.
Sun H, Sun Y, Chen Q, Xu Z. LncRNA KCNQ1OT1 contributes to the progression and chemoresistance in acute myeloid leukemia by modulating Tspan3 through suppressing miR-193a-3p. Life Sci. 2020; 241:117161. https://doi.org/10.1016/j.lfs.2019.117161 [PubMed]
-
21.
Yang C, Wu K, Wang S, Wei G. Long non-coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR-195-5p. J Cell Biochem. 2018; 119:5646–56. https://doi.org/10.1002/jcb.26743 [PubMed]
-
22.
Pop S, Enciu AM, Necula LG, Tanase C. Long non-coding RNAs in brain tumours: focus on recent epigenetic findings in glioma. J Cell Mol Med. 2018; 22:4597–610. https://doi.org/10.1111/jcmm.13781 [PubMed]
-
23.
Cao C, Xu Y, Du K, Mi C, Yang C, Xiang L, Xie Y, Liu W. LINC01303 functions as a competing endogenous RNA to regulate EZH2 expression by sponging miR-101-3p in gastric cancer. J Cell Mol Med. 2019; 23:7342–48. https://doi.org/10.1111/jcmm.14593 [PubMed]
-
24.
Kong L, Wu Q, Zhao L, Ye J, Li N, Yang H. Identification of messenger and long noncoding RNAs associated with gallbladder cancer via gene expression profile analysis. J Cell Biochem. 2019; 120:19377–87. https://doi.org/10.1002/jcb.28953 [PubMed]
-
25.
Yu X, Zheng H, Tse G, Zhang L, Wu WK. CASC2: an emerging tumour-suppressing long noncoding RNA in human cancers and melanoma. Cell Prolif. 2018; 51:e12506. https://doi.org/10.1111/cpr.12506 [PubMed]
-
26.
Xu R, Feng F, Yu X, Liu Z, Lao L. LncRNA SNHG4 promotes tumour growth by sponging miR-224-3p and predicts poor survival and recurrence in human osteosarcoma. Cell Prolif. 2018; 51:e12515. https://doi.org/10.1111/cpr.12515 [PubMed]
-
27.
Xiong WC, Han N, Wu N, Zhao KL, Han C, Wang HX, Ping GF, Zheng PF, Feng H, Qin L, He P. Interplay between long noncoding RNA ZEB1-AS1 and miR-101/ZEB1 axis regulates proliferation and migration of colorectal cancer cells. Am J Transl Res. 2018; 10:605–17. [PubMed]
-
28.
She K, Yan H, Huang J, Zhou H, He J. miR-193b availability is antagonized by LncRNA-SNHG7 for FAIM2-induced tumour progression in non-small cell lung cancer. Cell Prolif. 2018; 51:e12406. https://doi.org/10.1111/cpr.12406 [PubMed]
-
29.
Pan Y, Wu Y, Hu J, Shan Y, Ma J, Ma H, Qi X, Jia L. Long noncoding RNA HOTAIR promotes renal cell carcinoma Malignancy through alpha-2, 8-sialyltransferase 4 by sponging microRNA-124. Cell Prolif. 2018; 51:e12507. https://doi.org/10.1111/cpr.12507 [PubMed]
-
30.
Li Z, Li X, Chen C, Li S, Shen J, Tse G, Chan MT, Wu WK. Long non-coding RNAs in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2018; 51:e12483. https://doi.org/10.1111/cpr.12483 [PubMed]
-
31.
Wang L, Han S, Jin G, Zhou X, Li M, Ying X, Wang L, Wu H, Zhu Q. Linc00963: a novel, long non-coding RNA involved in the transition of prostate cancer from androgen-dependence to androgen-independence. Int J Oncol. 2014; 44:2041–49. https://doi.org/10.3892/ijo.2014.2363 [PubMed]
-
32.
Wu JH, Tian XY, An QM, Guan XY, Hao CY. LINC00963 promotes hepatocellular carcinoma progression by activating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2018; 22:1645–52. https://doi.org/10.26355/eurrev_201803_14574 [PubMed]
-
33.
Jiao H, Jiang S, Wang H, Li Y, Zhang W. Upregulation of LINC00963 facilitates melanoma progression through miR-608/NACC1 pathway and predicts poor prognosis. Biochem Biophys Res Commun. 2018; 504:34–39. https://doi.org/10.1016/j.bbrc.2018.08.115 [PubMed]
-
34.
Wang J, Li C, Xu L, Yang C, Zhang X. MiR-1193 was sponged by LINC00963 and inhibited cutaneous squamous cell carcinoma progression by targeting SOX4. Pathol Res Pract. 2019; 215:152600. https://doi.org/10.1016/j.prp.2019.152600 [PubMed]
-
35.
Liu HF, Zhen Q, Fan YK. LINC00963 predicts poor prognosis and promotes esophageal cancer cells invasion via targeting miR-214-5p/RAB14 axis. Eur Rev Med Pharmacol Sci. 2020; 24:164–73. https://doi.org/10.26355/eurrev_202001_19907 [PubMed]
-
36.
Liu W, Yang YJ, An Q. LINC00963 promotes ovarian cancer proliferation, migration and EMT via the miR-378g / CHI3L1 axis. Cancer Manag Res. 2020; 12:463–73. https://doi.org/10.2147/CMAR.S229083 [PubMed]
-
37.
Zhang N, Zeng X, Sun C, Guo H, Wang T, Wei L, Zhang Y, Zhao J, Ma X. LncRNA LINC00963 promotes tumorigenesis and radioresistance in breast cancer by sponging miR-324-3p and inducing ACK1 expression. Mol Ther Nucleic Acids. 2019; 18:871–81. https://doi.org/10.1016/j.omtn.2019.09.033 [PubMed]
-
38.
Schwind S, Maharry K, Radmacher MD, Mrózek K, Holland KB, Margeson D, Whitman SP, Hickey C, Becker H, Metzeler KH, Paschka P, Baldus CD, Liu S, et al. Prognostic significance of expression of a single microRNA, miR-181a, in cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol. 2010; 28:5257–64. https://doi.org/10.1200/JCO.2010.29.2953 [PubMed]
-
39.
Langer C, Marcucci G, Holland KB, Radmacher MD, Maharry K, Paschka P, Whitman SP, Mrózek K, Baldus CD, Vij R, Powell BL, Carroll AJ, Kolitz JE, et al. Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol. 2009; 27:3198–204. https://doi.org/10.1200/JCO.2008.20.6110 [PubMed]
-
40.
Hackanson B, Bennett KL, Brena RM, Jiang J, Claus R, Chen SS, Blagitko-Dorfs N, Maharry K, Whitman SP, Schmittgen TD, Lübbert M, Marcucci G, Bloomfield CD, Plass C. Epigenetic modification of CCAAT/enhancer binding protein alpha expression in acute myeloid leukemia. Cancer Res. 2008; 68:3142–51. https://doi.org/10.1158/0008-5472.CAN-08-0483 [PubMed]
-
41.
Chen C, Wang P, Mo W, Zhang Y, Zhou W, Deng T, Zhou M, Chen X, Wang S, Wang C. lncRNA-CCDC26, as a novel biomarker, predicts prognosis in acute myeloid leukemia. Oncol Lett. 2019; 18:2203–11. https://doi.org/10.3892/ol.2019.10591 [PubMed]
-
42.
Ke SD, Zhou XF. LncRNA MVIH knockdown inhibits the malignancy progression through downregulating miR-505 mediated HMGB1 and CCNE2 in acute myeloid leukemia. Translational cancer research. 2019; 8:2526. https://doi.org/10.21037/tcr.2019.10.12
-
43.
Wang K, Dai J, Liu T, Wang Q, Pang YX. LncRNA ZEB2-AS1 regulates the drug resistance of acute myeloid leukemia via the miR-142-3p/INPP4B axis. Rsc Adv. 2019; 9:39495–504. https://doi.org/10.1039/C9RA07854A
-
44.
Wu Z, Wang W, Wang Y, Wang X, Sun S, Yao Y, Zhang Y, Ren Z. Long noncoding RNA LINC00963 promotes breast cancer progression by functioning as a molecular sponge for microRNA-625 and thereby upregulating HMGA1. Cell Cycle. 2020; 5:6100–624. https://doi.org/10.1080/15384101.2020.1728024 [PubMed]
-
45.
Zhou Y, Yin L, Li H, Liu LH, Xiao T. The LncRNA LINC00963 facilitates osteosarcoma proliferation and invasion by suppressing miR-204-3p/FN1 axis. Cancer Biol Ther. 2019; 20:1141–48. https://doi.org/10.1080/15384047.2019.1598766 [PubMed]
-
46.
Ba Z, Gu L, Hao S, Wang X, Cheng Z, Nie G. Downregulation of lncRNA CASC2 facilitates osteosarcoma growth and invasion through miR-181a. Cell Prolif. 2018; 51:e12409. https://doi.org/10.1111/cpr.12409 [PubMed]
-
47.
Tian C, Deng Y, Jin Y, Shi S, Bi H. Long non-coding RNA RNCR3 promotes prostate cancer progression through targeting miR-185-5p. Am J Transl Res. 2018; 10:1562–70. [PubMed]
-
48.
Xue F, Che H. The long non-coding RNA LOC285758 promotes invasion of acute myeloid leukemia cells by down-regulating miR-204-5p. FEBS Open Bio. 2020; 10:734–43. https://doi.org/10.1002/2211-5463.12814 [PubMed]
-
49.
Gan S, Ma P, Ma J, Wang W, Han H, Chen L, Li X, Wu F, Sun H. Knockdown of ZFAS1 suppresses the progression of acute myeloid leukemia by regulating microRNA-150/Sp1 and microRNA-150/myb pathways. Eur J Pharmacol. 2019; 844:38–48. https://doi.org/10.1016/j.ejphar.2018.11.036 [PubMed]
-
50.
Dong X, Fang Z, Yu M, Zhang L, Xiao R, Li X, Pan G, Liu J. Knockdown of long noncoding RNA HOXA-AS2 suppresses chemoresistance of acute myeloid leukemia via the miR-520c-3p/S100A4 axis. Cell Physiol Biochem. 2018; 51:886–96. https://doi.org/10.1159/000495387 [PubMed]