Secreted frizzled-related protein 3 was genetically and functionally associated with developmental dysplasia of the hip
Abstract
Background: Developmental dysplasia of the hip (DDH) is the most common joint disease in child orthopedics. Secreted Frizzled-Related Protein 3 (FRZB) plays an important role in joint development. however, no direct association between FRZB and DDH has been demonstrated.
Methods: Analysis of genotype distribution and allele frequency for detected single nucleotide polymorphisms (SNP) of FRZB was performed. FRZB expression was assayed in DDH joint tissues. Further experiments to identify the chondrogenic properties of FRZB were conducted. Potential upstream miRNAs for FRZB were assayed in DDH.
Results: Significant difference in genotype distribution for rs3768842 (OR=1.46, P=0.0081) and rs2242040 (OR=0.65, P=0.0067) was found. DDH joint tissues showed significantly higher FRZB expression. FRZB demonstrated chondrogenic and anti-hypertrophic properties in vitro. FRZB modulated cell adhesion pathway and cell spreading by regulating integrins expressions. Upstream miRNAs regulating FRZB expression were identified in DDH synovial fluid. Experiments indicated that downregulated miRNA-454 caused FRZB upregulation in DDH joint.
Conclusion: Dysregulated FRZB and its loci were associated with DDH. As a Wnt antagonist with chondrogenic properties, FRZB modulated cell adhesion pathway and cell spreading by regulating integrins expressions. FRZB in multiple DDH joint tissues might be mediated by the dysregulated miRNA expression profiles in the joint synovial fluid.
Introduction
Developmental dysplasia of the hip (DDH) is a common hip joint disease in child orthopedics. The incidence of DDH is approximately 1 in 1000 live births, with a higher ratio in women [1, 2]. DDH broadly encompasses a spectrum of pathologic hip disorders in which hips are unstable, mainly including congenital dislocation/subdislocation of the hip and acetabular dysplasia in clinical. It is not only the concomitant physical appearance problems and activity limitation, but also the long-term complications such as hip osteoarthritis (OA) and femoral head necrosis that would severely influence the patients’ quality of life. The etiology of DDH has not been fully clarified. Multidisciplinary research indicated the environmental and genetic components of this disease. Although perinatal factors as breech presentation, high birthweight were considered to contribute to the occurrence of DDH [2], the important role of genetic factors cannot be neglected. Genes like TBX4, ASPN and GDF5 have been reported in DDH development in multiple populations [3–6], providing a good basis for genetic research on DDH.
Frizzled-related protein (FRZB) is a secreted Wnt antagonist [7–9]. FRZB was shown indispensable in chondrogenic differentiation of stem cells and could prevent hypertrophic differentiation of articular chondrocytes [10]. Cartilage integrity loss was found in Frzb-knockout mice, causing increased cartilage damage with age [11]. The important role for FRZB in joint homeostasis was further supported by shifted extracellular matrix components and decreased chondrocyte proliferation in Frzb-knockout mice [11, 12]. Polymorphisms rs288326 and rs7775 in gene FRZB were found to be associated with hip OA [13–16]. A case-control study of 451 Caucasian women with hip OA demonstrated that rs288326 and rs7775 in FRZB gene were also related to the proximal femur shape, and the presence of rs288326 even mediated the association between hip shape and OA [14]. Given the significance of FRZB in joint development, we assumed that FRZB might be involved in DDH development.
In current study, we report dysregulated FRZB and its loci in association with DDH. Our findings suggest that FRZB, as a Wnt antagonist with chondrogenic properties, modulated cell adhesion pathway and cell spreading by regulating integrins expressions. Moreover, FRZB in multiple DDH joint tissues might be mediated by the dysregulated miRNA expression profiles in the joint synovial fluid.
Materials and Methods
Patient collection and SNP genotyping in DDH patients and control
Between March 2014 and May 2016, a total of 386 patients with DDH retrieved from the department of orthopedic surgery and 558 healthy controls retrieved from physical examination department in the first affiliated hospital of Soochow University were enrolled to perform a case-control association study. All cases were confirmed to have unilateral or bilateral DDH (diagnosed according to clinical criteria and radiographic evidence). All controls had no symptom or history of DDH. Patients who had any systematic syndrome were excluded from the study. The study was approved by the ethical committee of the Soochow University, and written informed consent was obtained from the study subjects, whereby enrolled minors/children in the study would be signed by the guardians’ representative.
Genomic DNA was isolated from all the subjects either from buccal swabs with the DNA IQ System (Promega) or the peripheral blood with the NucleoSpin Kit (Macherey-Nagel GmbH and Co. KG). A case-control association study was performed by lab technician blinded to the sample status. SNPs in the current study were tag SNPs selected with the Haploview software (version 4.2). These loci were subsequently genotyped by Taqman assay both for cases and controls. Genetic analysis, data retrieval and the statistical processing are independently completed and reviewed by two persons. To ensure the accuracy of genotyping, 5% of the studied subjects were randomly selected for duplication and they had 100% concordance, indicating reliability of genotyping results.
FRZB expression in tissue samples of DDH patients and controls
Total RNA in clinical samples was isolated using RNA purification kits (ThermoFisher Scientific). Control samples were collected from patients with femoral head fracture or destructive amputation injury, and tissue samples of the hip joint were retrieved in the hip arthroplasty surgery or amputation surgery. A total 2 mg RNA was reverse transcribed to cDNA using a cDNA Reverse Transcription Kit (Applied Biosystems). FRZB expression in the tissue was measured by real-time PCR with gene-specific primers as follows: forward 5’-CTCATCAAGTACCGCCACTCGTG -3’, reverse 5’- CCGGAAATAGGTCTTCTGTGTAGCTC -3’ for the FRZB gene, and forward 5’-GAGTC AACGGATTTGGTCGT-3’, reverse 5’-TTGATTTTGGAGGGATCTCG-3’ for the endogenous control gene GAPDH. Real-time PCR was done as previously reported [17].
Immunofluorescence analysis of tissue sections
Immunofluorescence analysis of tissue sections was done as we previously described [17]. Briefly, sections were fixed in 4% PFA, washed with TBS-T, and then incubated with hyaluronidase (Sigma). After blocking with goat serum, sections were incubated with primary antibody for 2h at room temperature. PFA-embedded sections were deparaffinized and incubated in 1mM EDTA at 80° C for 15 mins for antigen retrieval. Primary antibodies for FRZB were diluted 1:200 and used (Abcam, Catalog No: ab273582). Immune complexes were detected with immunofluorescent secondary antibody using Goat Anti-Rabbit IgG H&L Alexa Fluor® 555 (Abcam, Catalog No. ab150078). DAPI (Beyotime biotechnology, Catalog No. C1002) was also used to detect the nucleus.
Different treatment of cultured BMSC and ATDC5 in vitro
BMSCs were acquired from mouse bone marrow as previously described [18]. Briefly, marrow aspirates (10-20 mL) were harvested and then transferred to plastic tubes. Retrieved BMSCs were further expanded in α-MEM medium [18]. Medium was changed every 2-3 days and Passage 2 BMSCs were used for the later experiments [19, 20]. For exogenous FRZB group, 50 ng/ml FRZB (R&D, Catalog No: AAB51298) was supplemented for two weeks into the medium. FRZB antibody (200ng/ml, Abcam, Catalog No: ab273587) was added into some cultures to neutralize FRZB in the medium. FRZB knockdown was performed using FRZB siRNA. FRZB siRNA (customized from GenePharma) and negative control(GenePharma, Catalog No. A06001) were acquired from GenePharma (Shanghai, China). Sequences used to construct FRZB-siRNA were 3’-GGAGATTCTAAAGTCCTCTTTCAAGAGAAGAGGACTTTAGAATCTC C-5’. The siRNAs were cloned into pGPU6/Neo (GenePharma). After confirmation of inhibiting efficacies, selected FRZB siRNA (150nM) or negative control was used to transfect primary ATDC5 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Isolated mRNAs were used to construct raw RNA sequence data obtained using an Illumina HiSeq™2000 machine [21]. The trimmed reads of mRNA profiles were further mapped with the Novoalign software (v2.07.11). mRNA expression profiles for the FRZB siRNA group and control group were profiled. Difference between FRZB siRNA and control group was analyzed using a paired two-sided test and the Bonferonni test. Fold changes and p-values were calculated for each mRNA profiled. Differentially enriched mRNAs were filtered with a fold change ≥ 2.0 and a false discovery rate (FDR) < 0.05 [21]. To predict the target pathways of differentially expressed mRNAs, Gene ontology (GO) categories of the predicted genes were analyzed using DAVID Bioinformatics Database, and pathway analysis (KEGG, Wiki, Reactome, Biocarta) was performed using the EnrichR web platform [21]. Validation of protein expressions were conducted with western blot for cells with different treatments as previously described. Anti-SOX9 antibody (Abcam, Catalog No: ab185966), Anti-ACAN antibody (Invitrogen, Catalog No: PA1-1746), Anti-ITGA8 antibody (Abcam, Catalog No: ab243027), Anti-ITGAV antibody (Abcam, Catalog No: ab179475) were used at the concentration of 1:200 dilution. Horseradish peroxidase (Jackson ImmunoLab) was used as the secondary antibody. GAPDH blots were used as control with GAPDH antibody (1:5000 diluted, Sigma).
Immunofluorescent analysis of ACAN (Invitrogen, Catalog No: PA1-1746) was performed to study the generated chondrocytes in different treatment. Images were observed with a confocal microscope (Leica, Japan). Expression of SOX9, Col2A1, GDF5 and Col10A1 after 2-week culture was quantified with RT-PCR. Chondrogenic tissues formed under different treatment was stained with alcian blue staining to detect proteoglycan production. Staining mages were taken with a light microscope (Leica Microsystems, Germany).
The expression of Wnt pathway genes(β-catenin, WNT3A and WNT8A), cartilage anabolic and catabolic markers (SOX9, Col1A1, Col2A1, MMP9, MMP13, ADAMTS5) and osteogenesis markers (Col10a1, RUNX2 and OCN) after 2-week culture was quantified and compared with RT-PCR. Primer sequences for genes in PCR assay were listed in Supplementary Table 1. Chondrogenesis was determined with alcian blue staining. GAG was quantitatively analyzed (6 vs 6) with normalization to DNA content. GAG content was compared among different treatment groups
microRNA expression and cell transfections
Total RNA was extracted from joint synovial fluid from DDH patients and control using Trizol reagent and then qRT-PCR was conducted with a SYBR Green qPCR Kit and the ABI Detection System. The expressions of miR-454, miR301a, miR301b, miR130a and miR130b were normalized to U6 applying the 2-ΔΔCt method. The primer details are listed in the supplementary file. MiRNA negative control (mimic control), miR-454 mimics or inhibitors were acquired from GenePharma. Transfection with these reagents was performed using Lipofectamine 3000 according to manufacturer’s protocol.
Statistics
SAS software (version 9.2) was applied to analyze the association between the SNPs and DDH occurrence. Odd ratios (ORs) and 95% confidence interval (CI) were reported. Two-sided chi-squared test was used to confirm the significance of differences in allelic distribution and P<0.05 was considered significantly different. Hardy-Weinberg equilibrium (HWE) was checked with chi-squared test in DDH patients and the control.
Results
Association between FRZB SNPs and the occurrence of DDH
Distribution of loci genotypes in both cases and controls conformed with HWE (p>0.05). Polymorphisms rs3768842 and rs2242070 were selected by Haploview software. Different allele frequency was noted between DDH patients and controls (p<0.05). Detailed distribution of genotype and alleles in DDH patients and healthy control has been listed (Tables 1, 2). An allele of rs3768842 was observed to have a positive association with the risk of DDH (OR=1.29, 95% CI: 1.07-1.56, p=0.0076), while the A allele of rs2242070 was found to have a negative association with the risk of DDH (OR=0.75, 95% CI: 0.62-0.90, p=0.0022). Significant difference was identified for genotype distribution (AA vs AG+GG) for rs3768842 (OR=1.46, 95% CI: 1.10-1.93, p=0.0081). For rs2242070, genotype distribution (AA vs AG+GG) also demonstrated significant difference (OR=0.65, 95% CI: 0.48-0.89, p=0.0067).
Table 1. Association between rs3768842 and DDH in different genetic models.
| Genotype | | Allele frequency | | OR (95%CI) | P value |
| AA | AG | GG | No. | A | G | A vs G | 1.29 (1.07-1.55) | 0.0076 |
| | | | | | |
| Case | 136 | 189 | 61 | 386 | 0.60 | 0.40 | AA vs others | 1.46 (1.10-1.93) | 0.0081 |
| | | | | | |
| Control | 151 | 293 | 112 | 556 | 0.54 | 0.46 | others vs GG | 1.34 (0.95-1.89) | 0.090 |
| | | | | | |
| OR = odds ratio, CI = confidence interval. |
Table 2. Association between rs2242070 and DDH in different genetic models.
| Genotype | | Allele frequency | | OR (95%CI) | P value |
| AA | AG | GG | No. | A | G | A vs G | 0.75 (0.62-0.90) | 0.0022 |
| | | | | | |
| Case | 79 | 202 | 104 | 386 | 0.47 | 0.53 | AA vs others | 0.65 (0.48-0.89) | 0.0067 |
| | | | | | |
| Control | 158 | 286 | 114 | 558 | 0.54 | 0.46 | others vs GG | 0.69 (0.51-0.94) | 0.0184 |
| | | | | | |
| OR = odds ratio, CI = confidence interval. |
Differential FRZB expression was demonstrated in DDH joint tissues
DDH patients showed higher expression of FRZB (Figure 1A) in joint tissue samples compared to the control. (3.72 ± 1.33 vs 6.21 ± 2.35, p = 0.003 for articular cartilage; 2.18 ±0.55 vs. 4.62 ± 1.73, p = 0.004 for joint ligament). Meanwhile, immunofluorescent analysis also showed greater FRZB expression in the DDH tissues. (Figure 1B)
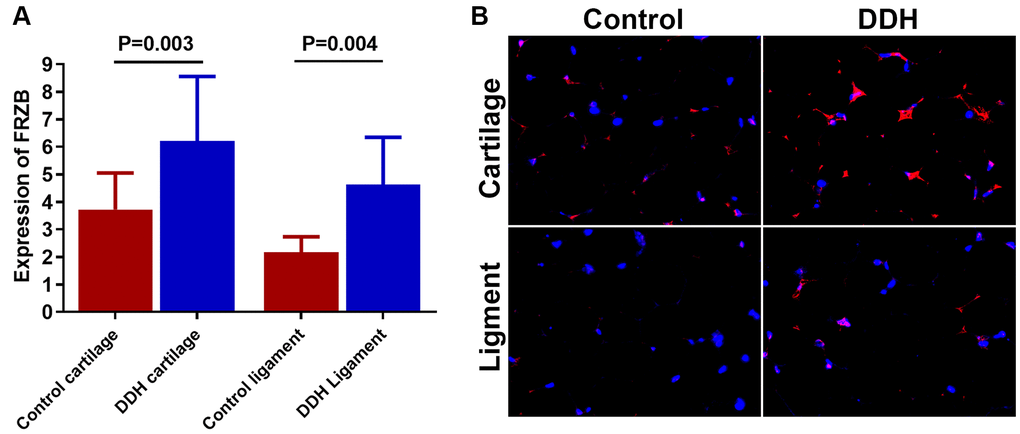
Figure 1. Tissue expression of FRZB in patients and controls. (A) DDH patients were found to have significantly higher expression of the FRZB in the articular cartilage and ligament as compared with the controls (2.43 ± 1.65 vs 4.05 ± 1.89, p = 0.002 for articular cartilage; 2.46 ±1.68 vs. 3.85 ± 2.73, p = 0.007 for joint ligament). (B) Immunofluorescent assay of FRZB (red) expression and nucleus (blue) in the cartilage and ligament tissues in different groups of patients observed under confocal microscopy.
FRZB induced chondrogenesis and Wnt signaling pathway in vitro in BMSCs
Effects of FRZB on chondrogenesis of BMSCs were examined in vitro. (Figure 2) exogenous recombinant FRZB enhanced GAG synthesis BMSCs, while medium supplemented with FRZB antibody decreased GAG deposition (Figure 2A). FRZB induced greater quantity of GAG deposition with higher expression of chondrogenic gene markers in the generated chondrogenic tissue (Figure 2B–2F). As a Wnt antagonist, FRZB blocked Wnt signaling by downregulating the expression of specific Wnt signaling genes (β-catenin, WNT3A and WNT8A). (Figure 3) Moreover, catabolic activities and osteogenesis of BMSCs were inhibited during the chondrogenic process induced by FRZB, indicative of non-hypertrophic chondrocytes induced by exogenous FRZB treatment (Figure 3).
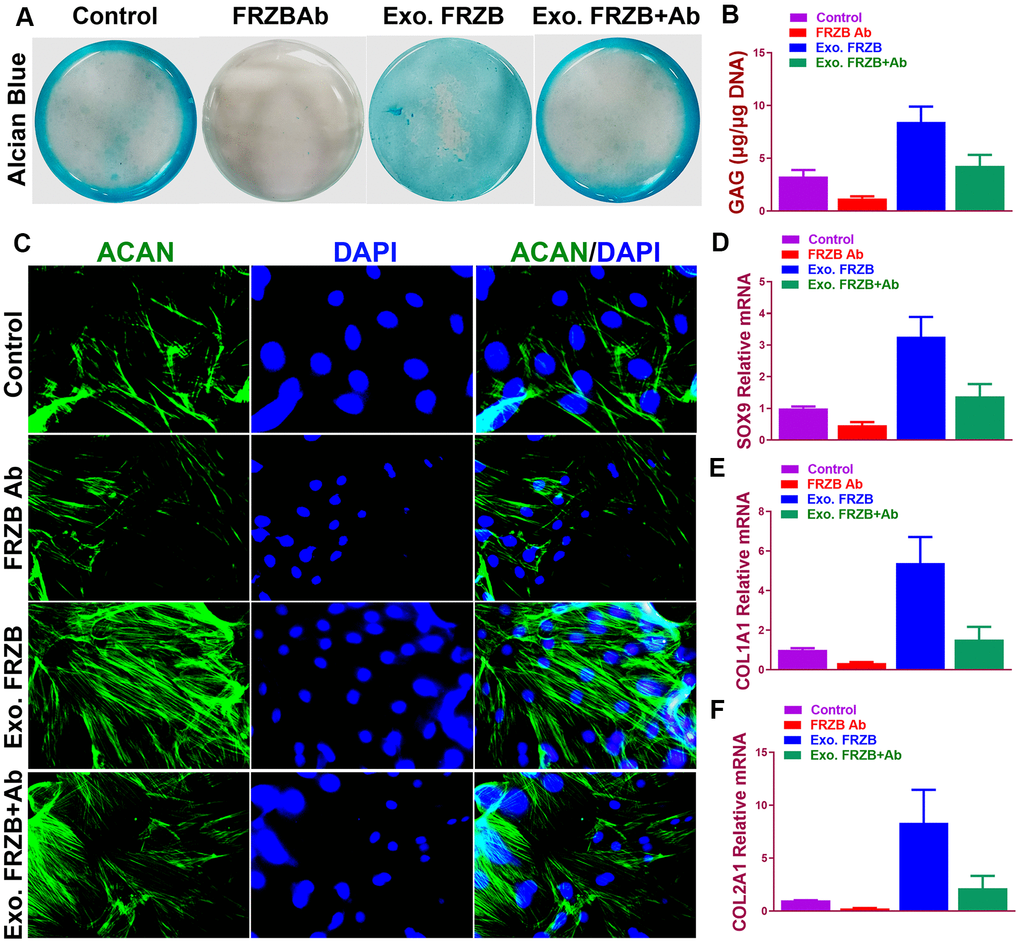
Figure 2. Chondrogenic effects of FRZB on BMSCs in vitro. (A) Alcian blue staining of BMSCs in different treatment groups at 2 weeks to indicate GAG production in the culture plate. (B) Quantification of GAG production in generated cartilaginous tissues (n=6 for each). (C) Immunofluorescent assay of Aggrecan (green) expression and nucleus (blue) in different treatment groups observed under confocal microscopy. (D–F) Expression level of chondrogenic markers for BMSCs in different treatment groups (n=6 for each) in (A) (n=6 for each). *P < 0.05 between control group and other groups. Data are presented as averages ± SD. One-way analysis of variance (ANOVA) with post-hoc Tukey’s B test was applied. Ab, antibody; Exo, exogenous; ACAN, aggrecan; GAG, glycosaminoglycan.
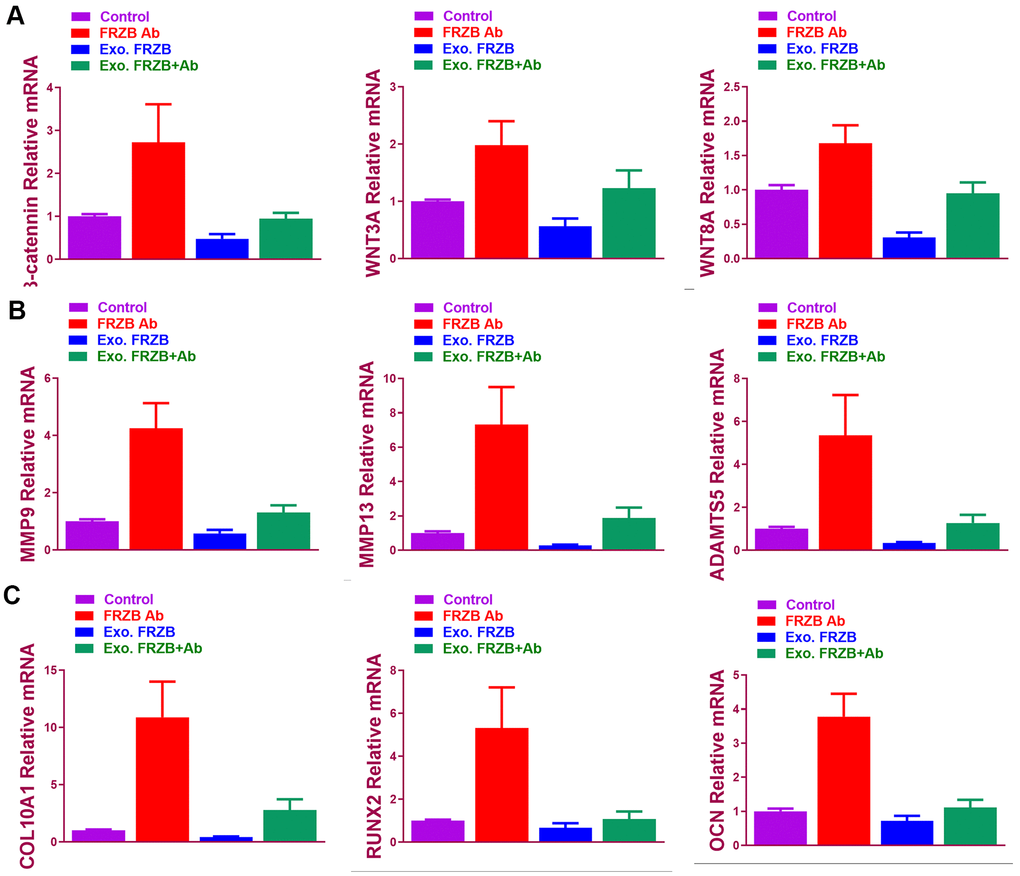
Figure 3. FRZB blocked Wnt signaling and inhibited the catabolic activities and hypertrophy in the induced chondrogenic tissues. (A) Expression level of Wnt pathway markers (β-catenin, WNT3A and WNT8A) for BMSCs in different treatment groups (n=6 for each) (B) Expression level of catabolic markers (MMP9, MMP13 and ADAMTS5) in different treatment groups (n=6 for each). (C) Expression level of hypertrophy and osteogenesis markers (COL10A1, RUNX2 and OCN) for BMSCs in different treatment groups (n=6 for each). *P < 0.05 between control group and other groups. Data are presented as averages ± SD. One-way analysis of variance (ANOVA) with post-hoc Tukey’s B test was applied.
Gene expression profiles in FRZB-knockdown ATDC5 cells
FRZB-knockdown was conducted with FRZB siRNA in chondrogenic progenitor ATDC5 cells. Enrichment of a set of mRNA microarray profiles in FRZB-knockdown ATDC5 cells was compared with control through mRNA sequencing (Figure 4A). Enriched mRNAs were filtered with a log2(fold change) ≥ 2.0 and a false discovery rate (FDR) < 0.05. Specifically, ASPN, COL3A1, CDH13, DKK3, LRRC15 and SFRP4 were up-regulated in FRZB-siRNA group, and ACAN, SOX9, DKK1, ARRB1, ITGAL and CDK1 were down-regulated compared with the control group (Figure 4A). Enrichments of the specific genes with FRZB-knockdown were further validated by qPCR and Western blot experiment, indicating significantly decreased chondrogenesis in FRZB-knockdown ATDC5 cells (Figure 4B, 4C). Gene ontology (GO) categories and KEGG analysis were performed with the differentially enriched mRNAs in FRZB-knockdown cells (Figure 4D, 4E). The enriched genes in FRZB-knockdown ATDC5 cells functioned in many biological processes such as response to stimulus, cell activation, extracellular matrix, cell adhesion molecular binding, signaling receptor binding and integrin binding. KEGG Pathway analysis results were closely associated with cellular adhesion molecules, AGE-RAGE signaling, Wnt signaling and focal adhesion. Protein-protein interaction network was demonstrated for enriched Wnt signaling pathway-related genes with FRZB-knockdown (Figure 4F).
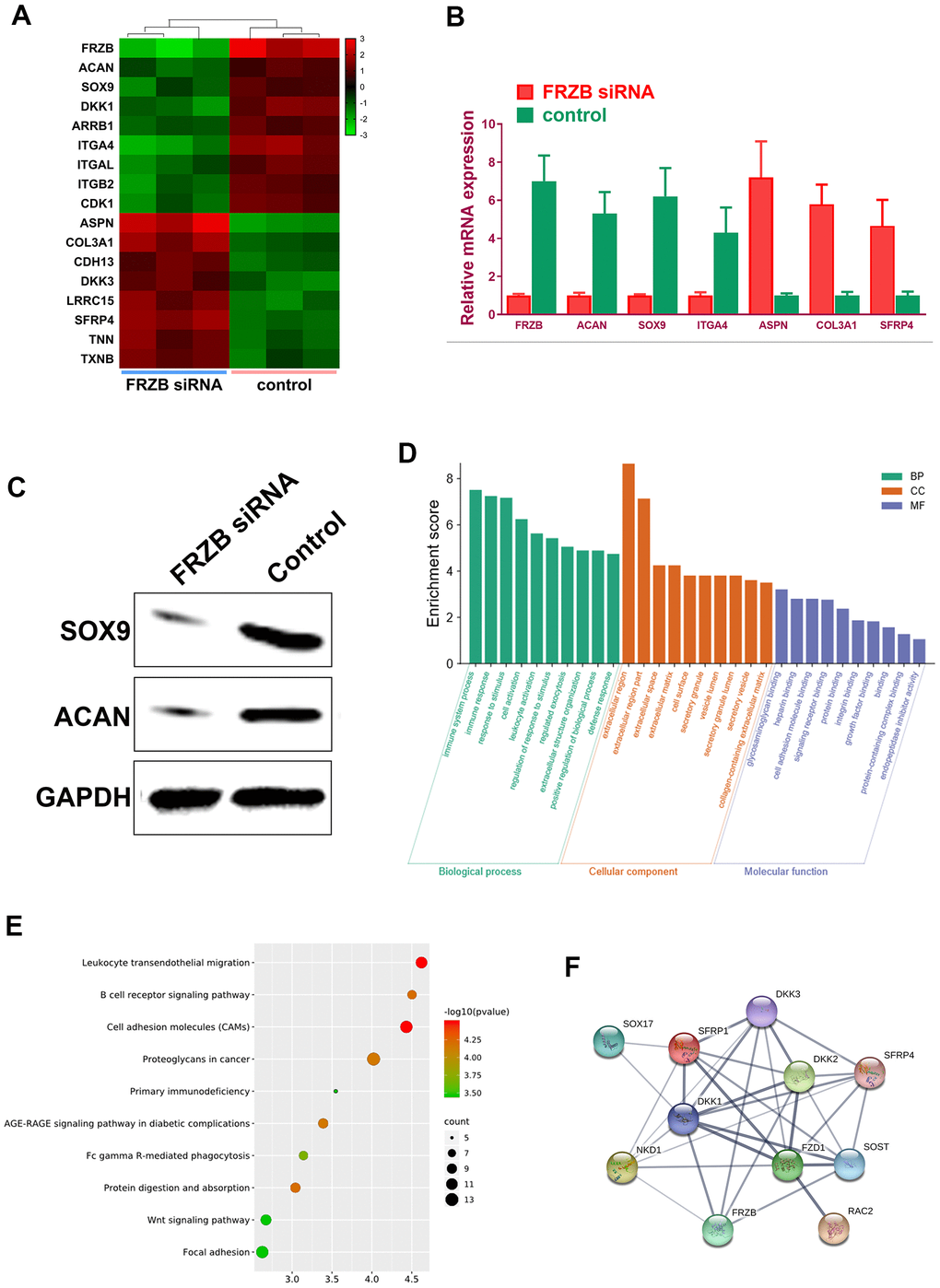
Figure 4. Specific mRNA microarray profiles in FRZB-knockdown ATDC5 cells. (A) The unique enrichment of a set of mRNA microarray profiles in FRZB-knockdown ATDC5 cells compared with control determined by high-throughput sequencing. Differentially enriched mRNAs were filtered with a log2(fold change) ≥ 2.0 and a false discovery rate (FDR) < 0.05. In detail, ASPN, COL3A1, CDH13, DKK3, LRRC15 and SFRP4 were significantly up-regulated in the FRZB-siRNA group, while ACAN, SOX9, DKK1, ARRB1, ITGAL and CDK1 were down-regulated compared with the control group. (B) The enrichments of specific gene expression with FRZB-knockdown were validated by qPCR. * P<0.05 vs. control. (C, D) Western blot experiment validated decreased chondrogenesis in FRZB-knockdown ATDC5 cells. (D, E) GO categories and KEGG pathway analysis were conducted based on the differentially enriched mRNAs in FRZB knockdown cells. The enriched mRNAs in FRZB-knockdown ATDC5 cells were involved in a broad range of biological functions, such as response to stimulus, cell activation, extracellular matrix, cell adhesion molecular binding, signaling receptor binding and integrin binding. KEGG Pathway analysis results were closely associated with cell adhesion molecules, AGE-RAGE signaling pathway, Wnt signaling pathway and focal adhesion. (F) Protein-protein interaction with enriched Wnt signaling pathway-related genes. Abbreviation: GO, gene ontology.
FRZB modulated cell adhesion pathway and cell spreading by regulating integrins expressions
Microarray profiles indicated enriched cell adhesion molecules and signaling in FRZB-knockdown cells (Figure 5A). In previous studies, dysregulated cell adhesion signal has been reported in osteoarthritis development. Taken together, cell adhesion signaling was analyzed in FRZB-knockdown ATDC5. Protein-protein interaction network was derived from the gene expression with enriched cell adhesion molecule and focal adhesion signaling pathway-related genes (Figure 5A). Integrin family members ITGA8 and ITGAV (red arrow) were enriched in both pathways. Western blot experiment further validated ITGA8 and ITGAV expression in FRZB-siRNA group (Figure 5B). Gene expressions of cell adhesion pathway genes were also validated and quantified with RT-PCR, showing significantly enhanced cell adhesion signaling and increased cell size with FRZB knockdown (Figure 5C–5E). Greater cell spreading was demonstrated in FRZB-knockdown cells with cytoskeleton staining (Figure 5D, 5E). To confirm the role of FRZB in cell adhesion and spreading, effects of exogenous FRZB and FRZB blockage on cell spreading was conducted (Figure 5F–5H). Exogenous FRZB significantly inhibited cell spreading while its blockage significantly enhanced cell spreading and increased cell size (Figure 5F). Exogenous FRZB significantly inhibited expression of related genes in cell adhesion signaling pathway while FRZB blockage exerted similar effects to FRZB knockdown in cell adhesion signaling (Figure 5G, 5H).
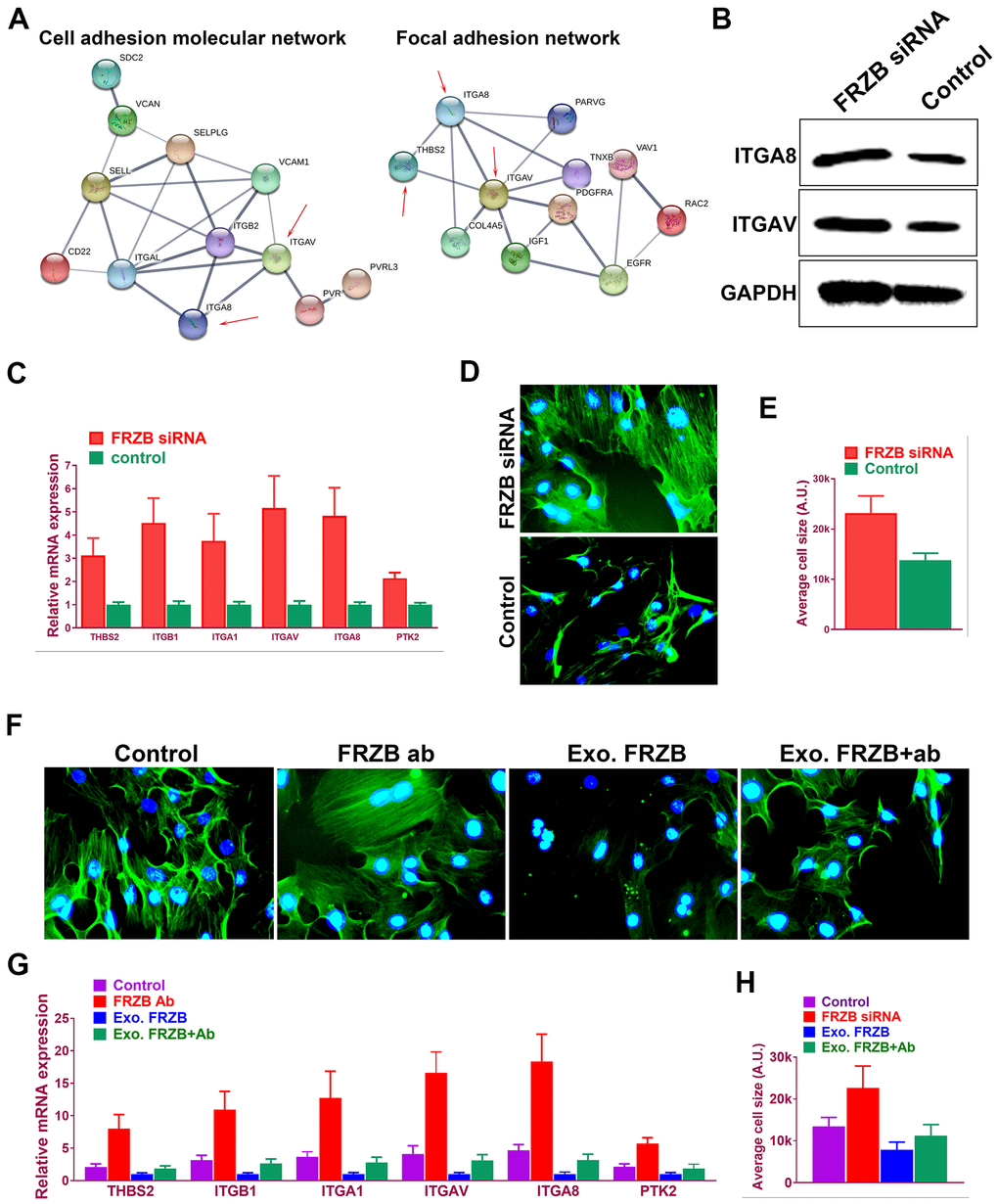
Figure 5. FRZB modulated cell adhesion pathway and cell spreading by regulating integrins expressions. (A) Protein-protein interaction with enriched cell adhesion molecule and focal adhesion signaling pathway-related genes. Integrin family members ITGA8 and ITGAV (red arrow) were enriched in both pathways. (B) Western blot experiment validated ITGA8 and ITGAV expression in FRZB-siRNA group. (C) Gene expressions of cell adhesion pathway genes were also validated and quantified with RT-PCR. (D, E) Greater cell spreading was demonstrated in FRZB-knockdown cells with cytoskeleton staining (green: phalloidin, blue: nucleus). (F, H) Effects of exogenous FRZB or FRZB blockage on cell spreading and (G) its regulation of related genes in cell adhesion signaling pathway.
Downregulated miRNA-454 expression causes FRZB upregulation in the synovial fluid of DDH patients
Our results had shown that FRZB acted as a pro-chondrogenic gene in multiple DDH joint tissues. Normally, we speculate that miRNAs conveyed in the synovial fluid in the DDH samples might involve in the upregulation of FRZB in the ligament and cartilage tissues. To explore the upstream miRNAs targeting FRZB, we queried the TargetScan, miRmap and miRanda and PITA databases. Five candidates emerged after overlapping potential miRNAs from the four databases (Figure 6A). We measured the expression of the five potential microRNA candidates in synovial fluid from DDH patients and found that miR-454, miR-301a and miR-301b were significantly downregulated in DDH SF compared with normal control samples (Figure 6B). Among the three dysregulated microRNAs, miR-454 was most significantly decreased in DDH SF, so we focused on miR-454 to explore its regulation of FRZB in vitro. Binding sites on FRZB for miR-454 was predicted with targetscan (Figure 6C). Sequence alignment of miR-454 showed high conservation among different species. ATDC5 were transfected with miR-454 mimics, miR-454 inhibitor or their negative control. MiR-454 mimics and inhibitor successfully up or down-regulated miR-454 expression in ATDC5 (Figure 6D). Additionally, PCR results also demonstrated that overexpression with miR-454 mimics could decrease FRZB expression while blockage of miR-454 increased FRZB expression (Figure 6E), indicating that the pro-chondrogenic effects of FRZB overexpression in DDH joint tissues might be mediated by the downregulation of miR-454 in the joint SF.
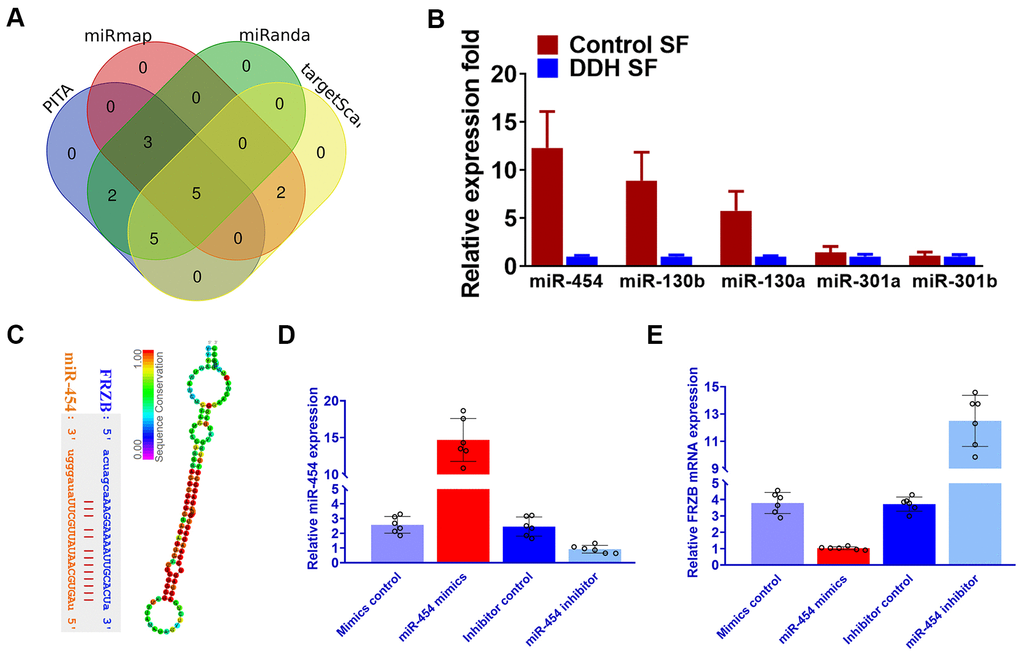
Figure 6. Downregulated miRNA-454 expression causes FRZB upregulation in the synovial fluid of DDH patients. (A) Five candidate miRNAs emerged after we queried the TargetScan, miRmap and miRanda and PITA databases. (B) Expression of the five microRNA candidates with qRT-PCR in synovial fluid from DDH patients compared with control samples. (C) Binding sites on FRZB for miR-454 (left panel) was predicted with targetscan and sequence alignment of miR-454 (right panel) showed high conservation among different species. (D) miR-454 expression by qRT-PCR in ATDC5 cells (n=6 for each) co-transfected with miR-454 mimics, miR-454 inhibitor or their negative control. (E) PCR results of FRZB expression with MiR-454 mimics and inhibitor in ATDC5.
Discussion
This study demonstrated significant associations between polymorphisms rs3768842 and rs2242070 in gene FRZB with DDH. Genotype distributions was significantly different (AA+AG vs GG) identified in cases and controls for both rs3768842 and rs2242070. DDH samples showed significantly greater FRZB expressions in DDH hip tissues compared to the control. FRZB also demonstrated chondrogenic and anti-hypertrophy properties as a wnt signaling antagonist, indicating that DDH might be attributed to the overdevelopment of femoral cartilage in patients with dysplastic hips. Potential upstream miRNAs regulating FRZB, miR-454 in specific, was identified in DDH joint synovial fluid, indicating FRZB overexpression in DDH joint tissues might be mediated by the dysregulation of miRNA expression profiles in the joint SF.
DDH is believed to be a polygenic disorder with increasing number of polymorphisms in various genes reported. Two SNPs of CX3CR1, rs3732378 (p=0.003) and rs3732379 (p=0.017) were identified as susceptibility loci of DDH through a case-control association study with 689 pairs of patients and controls [22]. Sun et al [23] conducted a genome-wide association study followed with a subsequent case-control study in two set of case-control groups, and identified risk allele A in rs6060373 of gene UQCC for DDH. However, it has to be noted that the positive findings of these genetic association studies should be interpreted with caution. A study on 310 patients with sporadic DDH and 487 controls performed by Jia et al [24] showed significant difference (p=0.001) of allele frequency in rs726252 of PAPPA2 gene and significantly different distribution of TT genotype between cases and controls (p=0.000). While another replication study found no significant difference between 697 DDH subjects and 707 controls in neither allele frequency nor genotype distribution of rs726252 [5].
Polymorphisms in WNT-signaling genes are found to be associated with osteoarthritis, suggesting its significance in pathological musculoskeletal conditions such as DDH [25, 26]. FRZB has been demonstrated to be a binding-gene in WNT signaling acting as a inhibition role, the protein encoded by which is a secreted protein to regulate skeletal development [27, 28]. A number of studies have shown FRZB to be a determinant of hip shape formation, whereby substitutions Arg200Trp and Arg324Gly in FRZB sequence conferred higher risks for OA development [14, 29, 30]. Despite no direct evidence supporting the association between expression of FRZB and the occurrence of DDH, the close link between primary hip OA and structural hip deformities makes FRZB a possible susceptibility gene worthy of study [31, 32]. Polymorphisms rs2242070 in FRZB gene was reported to be involved in the development of osteoporosis [33, 34]. While, this is the first study reporting polymorphisms rs2242070 and rs3768842 in gene FRZB to be associated with DDH, which suggests a potential role of FRZB in the occurrence of DDH. Expression of FRZB was found to be lost during OA progression, which can be used as markers for staging OA at the molecular level [35]. DDH cases normally had hip joint laxity in adolescence. FRZB was demonstrated indispensable in chondrogenesis and could induce cartilage overdevelopment in DDH, suggesting that dysregulated chondrogenesis in DDH could be mediated by FRZB signaling, leading to joint cartilage overdevelopment in the dysplastic hip joints. Further therapeutic approaches might be developed in the future by targeting the FRZB-mediated signaling or inhibiting the over-chondrogenesis of the dislocated hip joint
Our data suggest that FRZB inhibits cell adhesion and spreading by dampening integrin signaling in chondrogenic progenitors. Integrins are the major receptor to interact with the surrounding ECM of developing chondrocytes. Integrin-ECM
Integrin-ECM was previously reported in osteogenesis and inhibition of chondrogenesis [36]. Increased FAK activation inhibits chondrogenesis, while FAK is decreased in chondrocytes [37]. Furthermore, Cell-ECM has been intensively studied osteoarthritis occurrence. Cartilage injury would induce greater catabolism by MMP13 and ADAMTS5, causing further cartilage damage and releasing of ECM molecules into SF. ECM catabolite served as integrin ligands and further triggered catabolism of the articular tissues (23, 25). FRZB alleviates cartilage catabolism by inhibition of the activated integrins. significantly reducing cell spreading and focal adhesions. Further study is needed to address focal adhesion signaling in OA development. FRZB knockdown or blockage significantly reduced expression of chondrogenic markers, while exogenous FRZB was therapeutic and restored the chondrogenic phenotype. Integrin signal blockage has been tested for arthritis therapy (68–70). We have shown FRZB functions in chondrogenesis, and protects chondrocytes from destructive signals and integrin activation. FRZB is secreted and reduces integrin signaling by interacting with integrin. Experiments on FRZB function would shed light on new therapeutics for integrin-targeted diseases. Further replication studies on the association between FRZB and DDH might help early diagnosis of DDH in the future. Downregulation of miR-454 was demonstrated in DDH SF, and possibly caused the overexpression of FRZB in multiple DDH joint tissues. Dysregulation of miRNAs in the SF might be caused by the long-term joint dislocation, leading to a pro-chondrogenic microenvironment for the SMSC in the SF and further causing chondrogenic gene changes in the cartilage and other joint tissues. Further studies on the comprehensive miRNA expression profiles would help elicit how miRNAs and downstream chondrogenic genes were dysregulated in DDH development. There are some limitations to our study. GAPDH was used as a reference gene in our study as previously reported under the assumption that GAPDH expression was stable across tissue conditions [17, 38], but recent works have demonstrated that the expression of GAPDH is actually inconsistent [39]. When choosing a reference gene, it would be better to use other stable genes like PPIA or HPRT1 to crosscheck the expression of GAPDH and further validate the expression of the chondrogenesis-related genes mentioned in our study [40, 41]. Second, the two loci in our study were in the intron region of the FRZB gene. In this case, no association study was performed between loci genotype and the corresponding expression of FRZB. Further exploration of functional mutations in the promoter or exon regions would help uncover rare FRZB mutations responsible for abnormal FRZB expression in DDH patients.
Abbreviations
DDH: Developmental dysplasia of the hip;
FRZB: Frizzled-related protein;
OA: osteoarthritis;
SNP: Single nucleotide polymorphism;
OR: Odd ratios;
CI: confidence interval.
Author Contributions
Renjie Xu and Yuxing Zhang conceived the study. Renjie Xu and Fei Zhang conducted the experiments. Junlan Lu and Kexin Wang completed the collection and assembly of data. Peng Pan undertook the statistical analysis. Renjie Xu and Fei Zhang produced the initial draft manuscript. Fei Zhang, Ye Sun and Yuxin Zhang critically revised the article for important intellectual content and gave final approval of the article. All authors read the final manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
This study was funded by China National Natural Science Fund (No. 81802122) and the Fundamental research program funding of Ninth People’s Hospital affiliated to Shanghai Jiao Tong university School of Medicine (JYZZ078).
Editorial Note
&
This corresponding author has a verified history of publications using the personal email address for correspondence.
References
-
1.
Rosendahl K, Dezateux C, Fosse KR, Aase H, Aukland SM, Reigstad H, Alsaker T, Moster D, Lie RT, Markestad T. Immediate treatment versus sonographic surveillance for mild hip dysplasia in newborns. Pediatrics. 2010; 125:e9–16. https://doi.org/10.1542/peds.2009-0357 [PubMed]
-
2.
Chan A, McCaul KA, Cundy PJ, Haan EA, Byron-Scott R. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed. 1997; 76:F94–100. https://doi.org/10.1136/fn.76.2.F94 [PubMed]
-
3.
Wang K, Shi D, Zhu P, Dai J, Zhu L, Zhu H, Lv Y, Zhao B, Jiang Q. Association of a single nucleotide polymorphism in Tbx4 with developmental dysplasia of the hip: a case-control study. Osteoarthritis Cartilage. 2010; 18:1592–95. https://doi.org/10.1016/j.joca.2010.09.008 [PubMed]
-
4.
Shi D, Dai J, Zhu P, Qin J, Zhu L, Zhu H, Zhao B, Qiu X, Xu Z, Chen D, Yi L, Ikegawa S, Jiang Q. Association of the D repeat polymorphism in the ASPN gene with developmental dysplasia of the hip: a case-control study in Han Chinese. Arthritis Res Ther. 2011; 13:R27. https://doi.org/10.1186/ar3252 [PubMed]
-
5.
Shi D, Sun W, Xu X, Hao Z, Dai J, Xu Z, Chen D, Teng H, Jiang Q. A replication study for the association of rs726252 in PAPPA2 with developmental dysplasia of the hip in Chinese Han population. Biomed Res Int. 2014; 2014:979520. https://doi.org/10.1155/2014/979520 [PubMed]
-
6.
Rouault K, Scotet V, Autret S, Gaucher F, Dubrana F, Tanguy D, El Rassi CY, Fenoll B, Férec C. Evidence of association between GDF5 polymorphisms and congenital dislocation of the hip in a Caucasian population. Osteoarthritis Cartilage. 2010; 18:1144–49. https://doi.org/10.1016/j.joca.2010.05.018 [PubMed]
-
7.
Guo Y, Xie J, Rubin E, Tang YX, Lin F, Zi X, Hoang BH. Frzb, a secreted Wnt antagonist, decreases growth and invasiveness of fibrosarcoma cells associated with inhibition of Met signaling. Cancer Res. 2008; 68:3350–60. https://doi.org/10.1158/0008-5472.CAN-07-3220 [PubMed]
-
8.
Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997; 88:757–66. https://doi.org/10.1016/S0092-8674(00)81922-4 [PubMed]
-
9.
Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997; 88:747–56. https://doi.org/10.1016/S0092-8674(00)81921-2 [PubMed]
-
10.
Zhong L, Huang X, Rodrigues ED, Leijten JC, Verrips T, El Khattabi M, Karperien M, Post JN. Endogenous DKK1 and FRZB regulate chondrogenesis and hypertrophy in three-dimensional cultures of human chondrocytes and human mesenchymal stem cells. Stem Cells Dev. 2016; 25:1808–17. https://doi.org/10.1089/scd.2016.0222 [PubMed]
-
11.
Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007; 56:4095–103. https://doi.org/10.1002/art.23137 [PubMed]
-
12.
Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004; 18:1222–37. https://doi.org/10.1210/me.2003-0498 [PubMed]
-
13.
Evangelou E, Chapman K, Meulenbelt I, Karassa FB, Loughlin J, Carr A, Doherty M, Doherty S, Gómez-Reino JJ, Gonzalez A, Halldorsson BV, Hauksson VB, Hofman A, et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 2009; 60:1710–21. https://doi.org/10.1002/art.24524 [PubMed]
-
14.
Baker-Lepain JC, Lynch JA, Parimi N, McCulloch CE, Nevitt MC, Corr M, Lane NE. Variant alleles of the Wnt antagonist FRZB are determinants of hip shape and modify the relationship between hip shape and osteoarthritis. Arthritis Rheum. 2012; 64:1457–65. https://doi.org/10.1002/art.34526 [PubMed]
-
15.
Rodriguez-Lopez J, Pombo-Suarez M, Liz M, Gomez-Reino JJ, Gonzalez A. Further evidence of the role of frizzled-related protein gene polymorphisms in osteoarthritis. Ann Rheum Dis. 2007; 66:1052–55. https://doi.org/10.1136/ard.2006.065938 [PubMed]
-
16.
Lories RJ, Boonen S, Peeters J, de Vlam K, Luyten FP. Evidence for a differential association of the Arg200Trp single-nucleotide polymorphism in FRZB with hip osteoarthritis and osteoporosis. Rheumatology (Oxford). 2006; 45:113–14. https://doi.org/10.1093/rheumatology/kei148 [PubMed]
-
17.
Xu R, Jiang X, Lu J, Wang K, Sun Y, Zhang Y. Genetic variant of COL11A2 gene is functionally associated with developmental dysplasia of the hip in Chinese Han population. Aging (Albany NY). 2020; 12:7694–703. https://doi.org/10.18632/aging.103040 [PubMed]
-
18.
Sun Y, You Y, Jiang W, Wu Q, Wang B, Dai K. Generating ready-to-implant anisotropic menisci by 3D-bioprinting protein-releasing cell-laden hydrogel-polymer composite scaffold. Appl Mater Today
. 2020; 18:100469. https://doi.org/10.1016/j.apmt.2019.100469
-
19.
Yi SW, Park JS, Kim HJ, Lee JS, Woo DG, Park KH. Multiply clustered gold-based nanoparticles complexed with exogenous pDNA achieve prolonged gene expression in stem cells. Theranostics. 2019; 9:5009–19. https://doi.org/10.7150/thno.34487 [PubMed]
-
20.
Lu J, Shen X, Sun X, Yin H, Yang S, Lu C, Wang Y, Liu Y, Huang Y, Yang Z, Dong X, Wang C, Guo Q, et al. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics. 2018; 8:5039–58. https://doi.org/10.7150/thno.26981 [PubMed]
-
21.
Jing H, Zhang X, Luo K, Luo Q, Yin M, Wang W, Zhu Z, Zheng J, He X. miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials. 2020; 231:119682. https://doi.org/10.1016/j.biomaterials.2019.119682 [PubMed]
-
22.
Li L, Wang X, Zhao Q, Wang E, Wang L, Cheng J, Zhang L, Wang B. CX3CR1 polymorphisms associated with an increased risk of developmental dysplasia of the hip in human. J Orthop Res. 2017; 35:377–80. https://doi.org/10.1002/jor.23294 [PubMed]
-
23.
Sun Y, Wang C, Hao Z, Dai J, Chen D, Xu Z, Shi D, Mao P, Teng H, Gao X, Hu Z, Shen H, Jiang Q. A common variant of ubiquinol-cytochrome c reductase complex is associated with DDH. PLoS One. 2015; 10:e0120212. https://doi.org/10.1371/journal.pone.0120212 [PubMed]
-
24.
Jia J, Li L, Zhao Q, Zhang L, Ru J, Liu X, Li Q, Shi L. Association of a single nucleotide polymorphism in pregnancy-associated plasma protein-A2 with developmental dysplasia of the hip: a case-control study. Osteoarthritis Cartilage. 2012; 20:60–63. https://doi.org/10.1016/j.joca.2011.10.004 [PubMed]
-
25.
Lodewyckx L, Lories RJ. WNT Signaling in osteoarthritis and osteoporosis: what is the biological significance for the clinician? Curr Rheumatol Rep. 2009; 11:23–30. https://doi.org/10.1007/s11926-009-0004-6 [PubMed]
-
26.
Luyten FP, Tylzanowski P, Lories RJ. Wnt signaling and osteoarthritis. Bone. 2009; 44:522–27. https://doi.org/10.1016/j.bone.2008.12.006 [PubMed]
-
27.
Bodine PV, Billiard J, Moran RA, Ponce-de-Leon H, McLarney S, Mangine A, Scrimo MJ, Bhat RA, Stauffer B, Green J, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005; 96:1212–30. https://doi.org/10.1002/jcb.20599 [PubMed]
-
28.
Killock D. Osteoarthritis: frzb knockout reveals the complexity of Wnt signaling in joint homeostasis. Nat Rev Rheumatol. 2012; 8:123. https://doi.org/10.1038/nrrheum.2012.15 [PubMed]
-
29.
Lane NE, Lian K, Nevitt MC, Zmuda JM, Lui L, Li J, Wang J, Fontecha M, Umblas N, Rosenbach M, de Leon P, Corr M. Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum. 2006; 54:1246–54. https://doi.org/10.1002/art.21673 [PubMed]
-
30.
Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, Ferreira A, Ciesielski C, Carson DA, Corr M. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA. 2004; 101:9757–62. https://doi.org/10.1073/pnas.0403456101 [PubMed]
-
31.
Stelzeneder D, Mamisch TC, Kress I, Domayer SE, Werlen S, Bixby SD, Millis MB, Kim YJ. Patterns of joint damage seen on MRI in early hip osteoarthritis due to structural hip deformities. Osteoarthritis Cartilage. 2012; 20:661–69. https://doi.org/10.1016/j.joca.2012.03.014 [PubMed]
-
32.
Okano K, Enomoto H, Osaki M, Shindo H. Outcome of rotational acetabular osteotomy for early hip osteoarthritis secondary to dysplasia related to femoral head shape: 49 hips followed for 10-17 years. Acta Orthop. 2008; 79:12–17. https://doi.org/10.1080/17453670710014699 [PubMed]
-
33.
Tranah GJ, Taylor BC, Lui LY, Zmuda JM, Cauley JA, Ensrud KE, Hillier TA, Hochberg MC, Li J, Rhees BK, Erlich HA, Sternlicht MD, Peltz G, Cummings SR, and Study of Osteoporotic Fractures (SOF) Research Group. Genetic variation in candidate osteoporosis genes, bone mineral density, and fracture risk: the study of osteoporotic fractures. Calcif Tissue Int. 2008; 83:155–66. https://doi.org/10.1007/s00223-008-9165-y [PubMed]
-
34.
Higuchi RG, Peltz GA, Fijal B, Ro SK, Li J. WO2004094659A1 Associations of polymorphisms in the FRZB gene in obesity and osteoporosis. Google Patents. 2010.
-
35.
Zhong L, Huang X, Karperien M, Post JN. Correlation between gene expression and osteoarthritis progression in human. Int J Mol Sci. 2016; 17:1126. https://doi.org/10.3390/ijms17071126 [PubMed]
-
36.
Tang Y, Rowe RG, Botvinick EL, Kurup A, Putnam AJ, Seiki M, Weaver VM, Keller ET, Goldstein S, Dai J, Begun D, Saunders T, Weiss SJ. MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis. Dev Cell. 2013; 25:402–16. https://doi.org/10.1016/j.devcel.2013.04.011 [PubMed]
-
37.
Pala D, Kapoor M, Woods A, Kennedy L, Liu S, Chen S, Bursell L, Lyons KM, Carter DE, Beier F, Leask A. Focal adhesion kinase/Src suppresses early chondrogenesis: central role of CCN2. J Biol Chem. 2008; 283:9239–47. https://doi.org/10.1074/jbc.M705175200 [PubMed]
-
38.
Sun Y, You Y, Jiang W, Zhai Z, Dai K. 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics. 2019; 9:6949–61. https://doi.org/10.7150/thno.38061 [PubMed]
-
39.
Zhai Z, Yao Y, Wang Y. Importance of suitable reference gene selection for quantitative RT-PCR during ATDC5 cells chondrocyte differentiation. PLoS One. 2013; 8:e64786. https://doi.org/10.1371/journal.pone.0064786 [PubMed]
-
40.
Tan SC, Ismail MP, Duski DR, Othman NH, Bhavaraju VM, Ankathil R. Identification of optimal reference genes for normalization of RT-qPCR data in cancerous and non-cancerous tissues of human uterine cervix. Cancer Invest. 2017; 35:163–73. https://doi.org/10.1080/07357907.2017.1278767 [PubMed]
-
41.
Tan SC. Use of arbitrary reference genes may lead to misleading conclusions. Gynecol Obstet Invest. 2019; 84:519–20. https://doi.org/10.1159/000501684 [PubMed]