The upregulated expression of RFC4 and GMPS mediated by DNA copy number alteration is associated with the early diagnosis and immune escape of ESCC based on a bioinformatic analysis
Abstract
Esophageal squamous cell carcinoma (ESCC) is a malignant tumor that commonly occurs worldwide. Usually, Asia, especially China, has a high incidence of esophageal cancer. ESCC often has a poor outcome because of a late diagnosis and lack of effective treatments.
To build foundations for the early diagnosis and treatment of ESCC, we used the gene expression datasets GSE20347 and GSE17351 from the GEO database and a private dataset to uncover differentially expressed genes (DEGs) and key genes in ESCC. Notably, we found that replication factor C subunit 4 (RFC4) and guanine monophosphate synthase (GMPS) were upregulated but have been rarely studied in ESCC. In particular, to the best of our knowledge, our study is the first to explore GMPS and ESCC. Furthermore, we found that high levels of RFC4 and GMPS expression may result from an increase in DNA copy number alterations. Furthermore, RFC4 and GMPS were both upregulated in the early stage and early nodal metastases of esophageal carcinoma. The expression of RFC4 was strongly correlated with GMPS. In addition, we explored the relationship between RFC4 and GMPS expression and tumor-infiltrating immune cells (TILs) in esophageal carcinoma. The results showed that the levels of RFC4 and GMPS increased with a decrease in some tumor-infiltrating cells. Upregulated RFC4 and GMPS with high TILs indicate a worse prognosis.
In summary, our study shows that RFC4 and GMPS have potential as biomarkers for the early diagnosis of ESCC and may played a crucial role in the process of tumor immunity in ESCC.
Introduction
Esophageal carcinoma is a common malignant tumor worldwide [1, 2]. This cancer ranked seventh in cancer incidence and sixth in mortality overall in 2018 [3]. Most cases of esophageal cancer, especially in Eastern Europe and Asia, are squamous cell carcinoma [3–5]. Usually, tobacco and alcohol consumption are major risk factors for esophageal carcinoma [6, 7]. Esophagectomy is the major therapy for locoregional esophageal cancer [8]. Although a multidisciplinary approach is used for esophageal cancer treatment, the prognosis of patients with esophageal carcinoma is still poor [9]. In fact, one of the main reasons is that esophageal cancer is usually diagnosed at a late stage [10]. Researchers also believe that an earlier diagnosis is associated with better outcomes than a late diagnosis [11, 12]. However, the lack of early diagnosis markers remains a great challenge for esophageal carcinoma treatment and prognosis [13].
In recent decades, bioinformatics has become an important component of cancer research [14]. In particular, increasing numbers of public datasets, such as The Gene Expression Omnibus (GEO) database and The Cancer Genome Atlas (TCGA) database, have been established for oncology research. Usually, researchers use these datasets to screen tumor-associated biomarkers and excavate potential genetic targets of cancer [15, 16].
In the present study, we focused our research on ESCC. GSE20347 and GSE17351 from GEO and one of our private datasets were used to identify differentially expressed genes (DEGs) in ESCC. Then, Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) and protein-protein interaction (PPI) network analyses were used to identify the relevant functions of the DEGs. Among the most significant DEGs, RFC4 and GMPS were both increased in the early stage and early nodal metastases of esophageal carcinoma. When the levels of RFC4 and GMPS increased, we found a decrease in some tumor-infiltrating cells. Therefore, we hypothesize that RFC4 and GMPS are involved in the early progression of esophageal cancer, and mediate the immune escape of esophageal carcinoma.
In summary, our results reveal that RFC4 and GMPS have potential as early diagnostic markers and new immunotherapy targets for ESCC. To date, our study is the first to systematically explore the functions of RFC4 and GMPS in ESCC. Furthermore, the discovery of RFC4 and GMPS can help us better understand the early diagnosis and treatment of esophageal cancer.
Results
Identification of DEGs, PPI network construction and hub gene selection in ESCC
Flow chart of the whole data analysis is shown in Figure 1A. According to the comparison of GSE20347, GSE17351 and our private dataset, there were 25 ESCC tissues and 25 normal tissues in the present study. Using GEO2R online tools and Venn diagram software, the results showed that in total, 64 genes were identified as DEGs (Figure 1B, 1C), including 22 downregulated genes and 42 upregulated genes in the ESCC tissues (Supplementary Table 1). Then, we used STRING and Cytoscape tools to construct the PPI network of the DEGs (Figure 1D) and screen the hub genes. The results obtained using the cytoHubba module of Cytoscape showed that 14 genes with a degree >4 were identified as hub genes (Figure 1E), including COL7A1, RFC4, MMP13, COL11A1, TOP2A, LAMB3, LAMC2, CENPF, MCM2, GMPS, CKS1B, ECT2, COL10A1, and ITGA6 (Table 1).
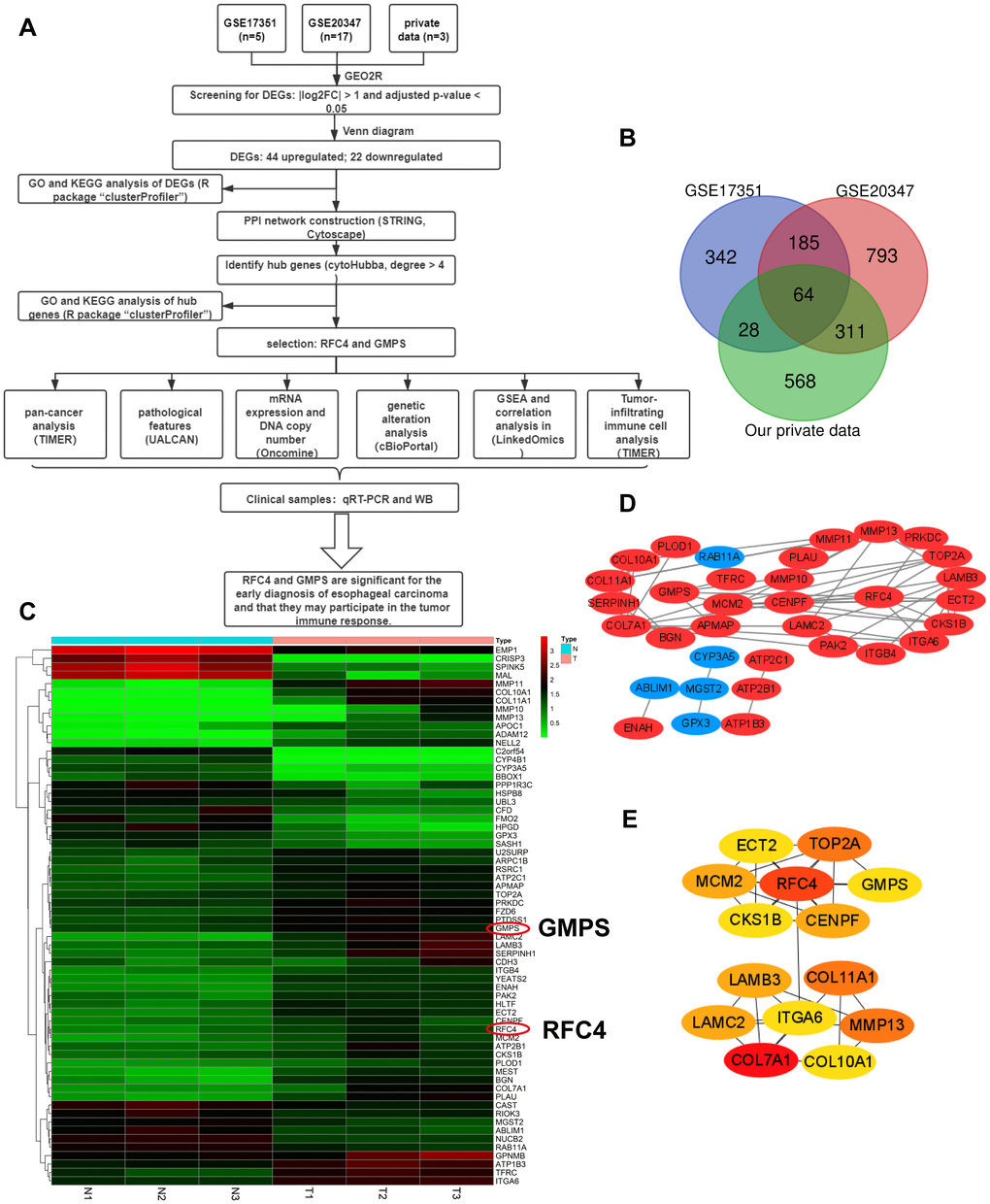
Figure 1. Differentially expressed genes analyzed in the GSE20347, GSE17351 and private datasets. (A) Flow chart of the data analysis in this study. (B) Venn diagrams of the DEGs from the GSE20347, GSE17351 and private datasets. (C) Heatmap of 64 DEGs from the private dataset. (D) Visual PPI network of 64 DEGs from Cytoscape. Upregulated genes are marked in red; downregulated genes are marked in blue. (E) Fourteen hub genes screened by a degree > 4.
Table 1. 14 hub genes.
| Gene symbol | Full name | Degree score |
| COL7A1 | Collagen alpha-1(VII) chain | 9 |
| RFC4 | Replication factor C subunit 4 | 8 |
| MMP13 | Collagenase 3 | 7 |
| COL11A1 | Collagen alpha-1(XI) chain | 7 |
| TOP2A | DNA topoisomerase 2-alpha | 7 |
| LAMB3 | Laminin subunit beta-3 | 6 |
| LAMC2 | Laminin subunit gamma-2 | 6 |
| CENPF | Centromere protein F | 6 |
| MCM2 | DNA replication licensing factor MCM2 | 6 |
| GMPS | guanine monophosphate synthase | 5 |
| CKS1B | Cyclin-dependent kinases regulatory subunit 1 | 5 |
| ECT2 | Protein ECT2 | 5 |
| COL10A1 | Collagen alpha-1(X) chain | 5 |
| ITGA6 | Integrin alpha-6 | 5 |
Functional analysis of DEGs and hub genes
To analyze the potential biological function and signaling pathways of the DEGs and hub genes, GO and KEGG pathway analyses were performed using the R package “clusterProfiler”.
Regarding the DEGs, the GO analysis results showed that the changes in the DEGs were significantly enriched in extracellular matrix structural constituents (Figure 2A). The KEGG pathway analysis revealed that the DEGs were mainly enriched in ECM-receptor interactions (Figure 2B).
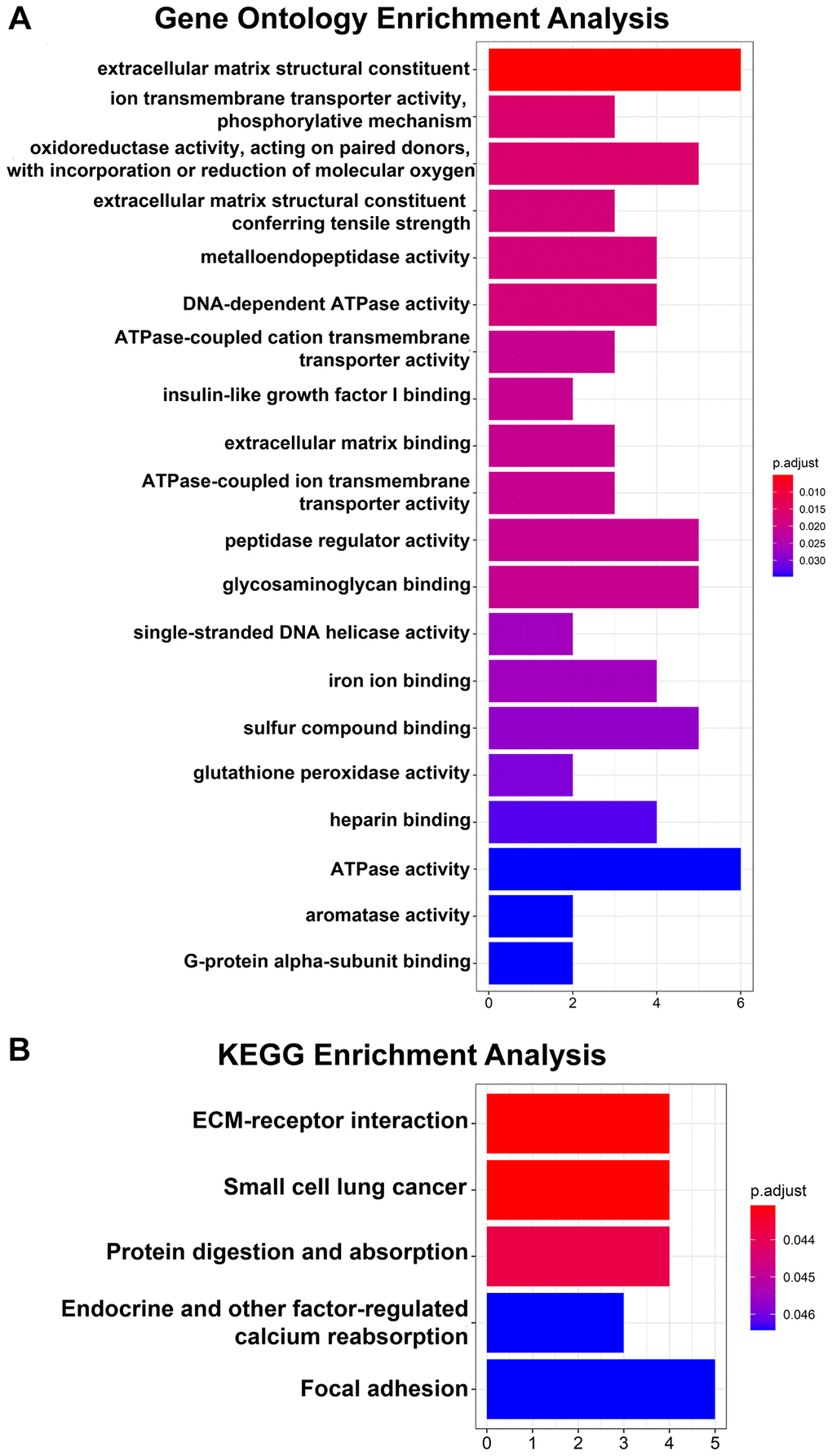
Figure 2. GO and KEGG pathway enrichment analyses of 64 DEGs. (A) Top 20 GO enrichment analyses of 64 DEGs. (B) KEGG pathway analysis of 64 DEGs. P < 0.05 indicates statistical significance.
Regarding the hub genes, the GO analysis results indicated that the hub genes were mainly enriched in extracellular matrix structural constituents, extracellular matrix structural constituents conferring tensile strength and DNA-dependent ATPase activity and so on (Figure 3A). Moreover, the KEGG pathway analysis revealed that the hub genes were enriched in small cell lung cancer, ECM-receptor interaction and protein digestion and adsorption (Figure 3B).
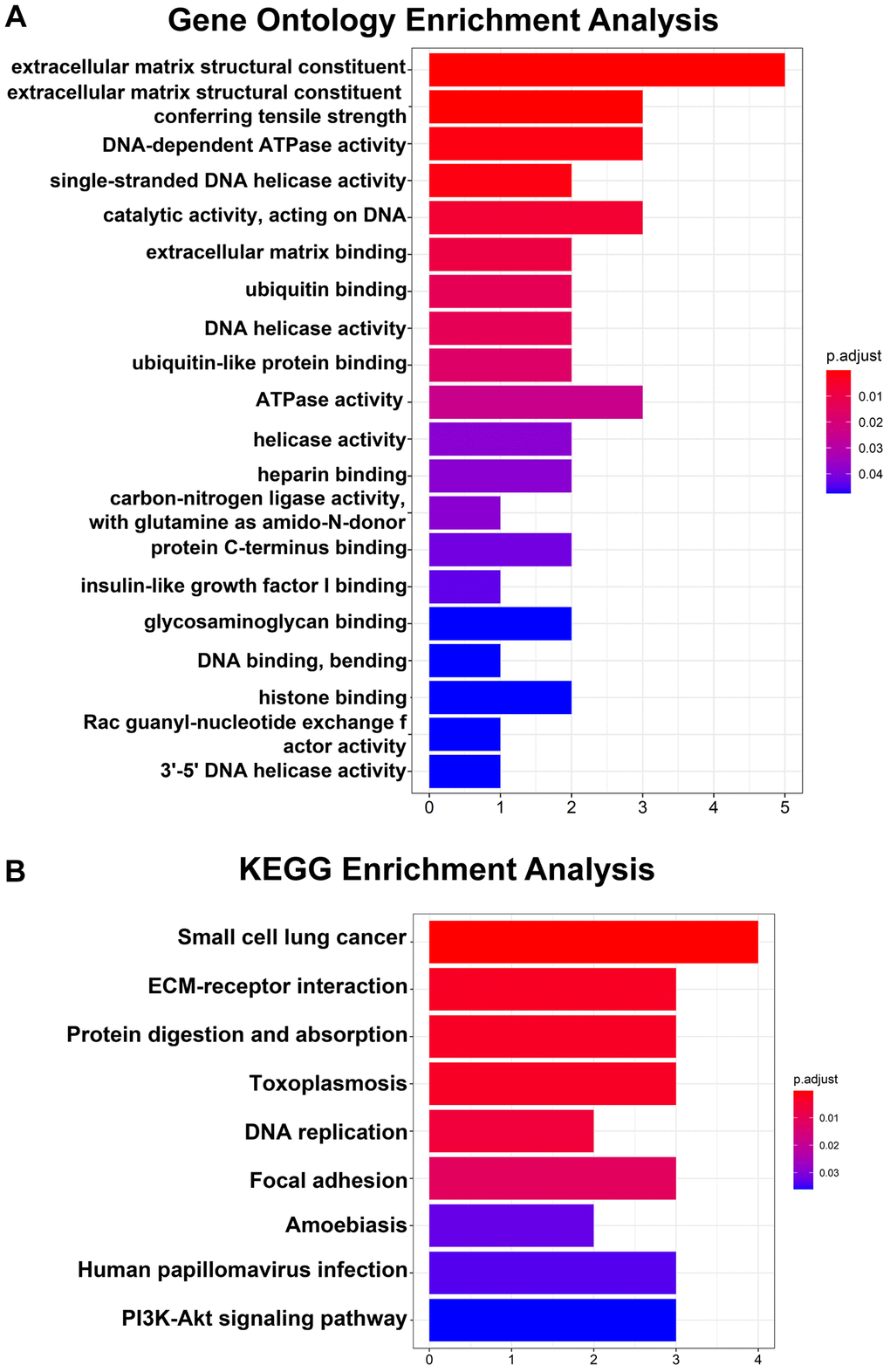
Figure 3. GO and KEGG pathway analyses of 14 hub genes. (A) Top 20 GO enrichment analyses of 14 hub genes. (B) KEGG pathway analysis of 14 hub genes. P < 0.05 indicates statistical significance.
Meanwhile, by examining the data, we found that RFC4 and GMPS have rarely been reported in ESCC. In particular, to the best of our knowledge, our study is the first to report the relationship between GMPS and ESCC. Therefore, we focused our attention on RFC4 and GMPS in ESCC.
RFC4 and GMPS were upregulated in cancers, especially ESCC
By conducting an online TCGA analysis, we found that the expression of RFC4 and GMPS was upregulated in many cancers (Figure 4A, 4B). Then, we focus our attention on ESCA. By conducting a UALCAN online analysis, the results indicated that RFC4 and GMPS were increased significantly in esophageal carcinoma compared to normal tissues, especially in esophageal squamous cell carcinoma (Figure 4C, 4D).
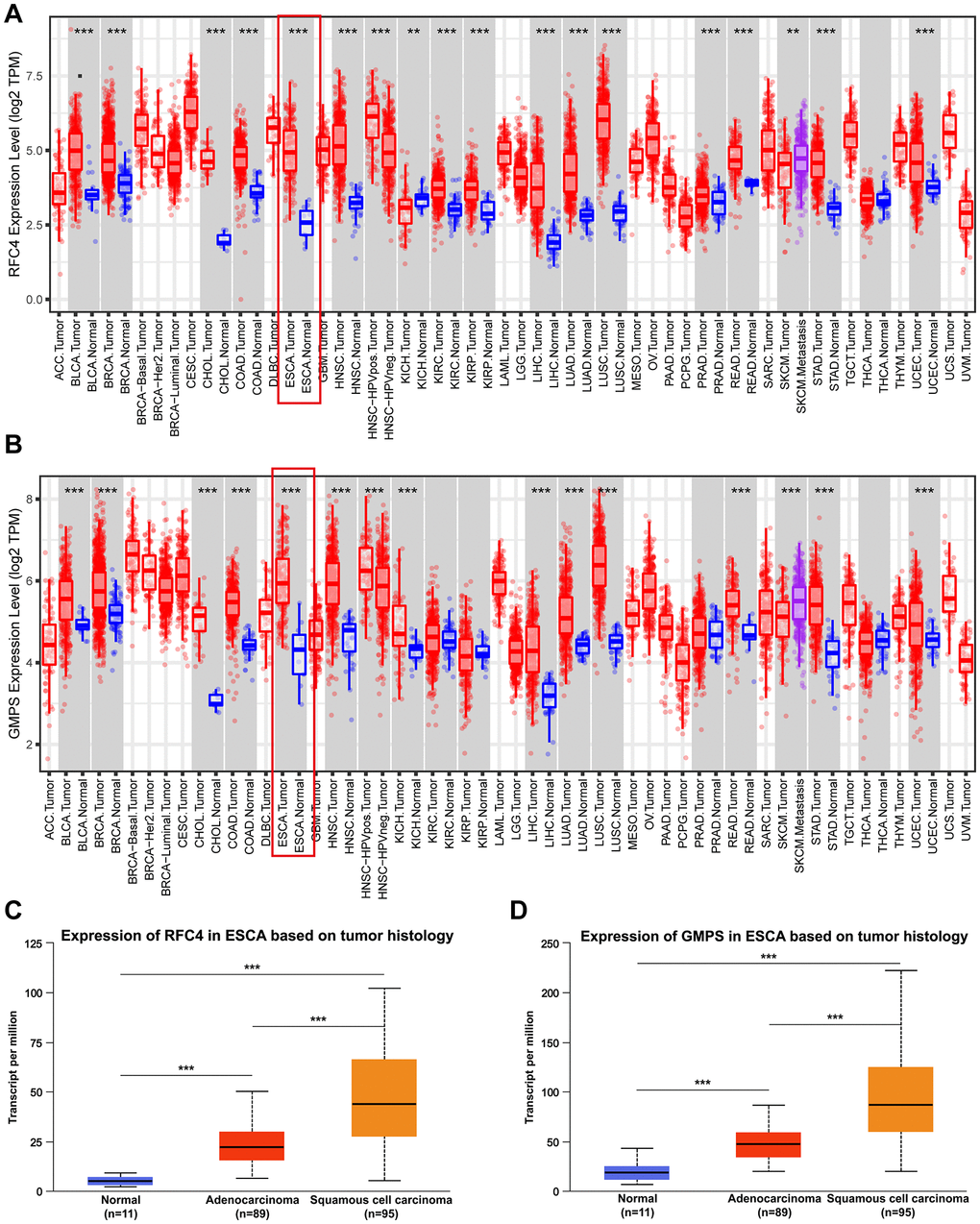
Figure 4. RFC4 and GMPS were upregulated in most cancers, especially ESCC. (A) Pan-cancer analysis of RFC4. (B) Pan-cancer analysis of GMPS. (C) Expression of RFC4 in patients with esophageal carcinoma based on histology. (D) Expression of GMPS in patients with esophageal carcinoma based on histology. *p < 0.05, **p < 0.01, ***p < 0.001.
In order to further explore the expression of RFC4 and GMPS in the esophageal carcinoma, the Oncomine dataset was used. We found that RFC4 and GMPS were both increased in the two different ESCC datasets (Su and Hu Esophagus datasets) [17, 18] (Figure 5A–5F).
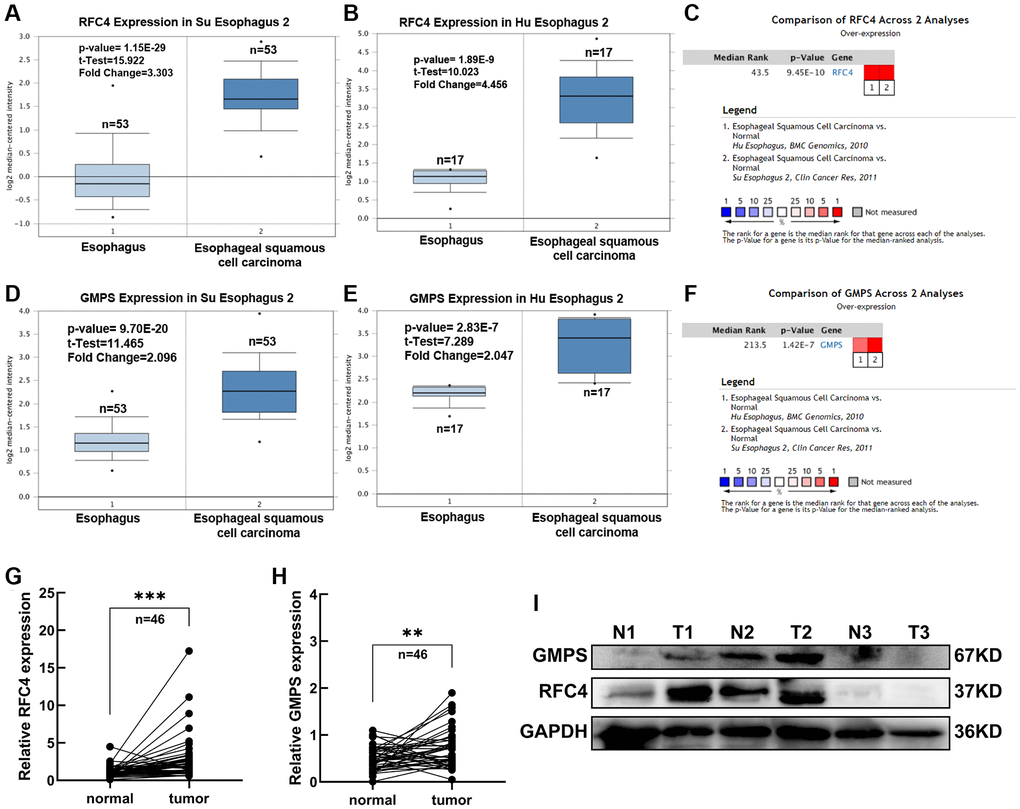
Figure 5. Expression analysis of RFC4 and GMPS in ESCC. (A) RFC4 mRNA levels in Su Esophagus 2. (B) RFC4 mRNA levels in Hu Esophagus 2. (C) Comparison of RFC4 across Su and Hu Esophagus. (D) GMPS mRNA levels in Su Esophagus 2. (E) GMPS mRNA levels in Hu Esophagus 2. (F) Comparison of GMPS across Su and Hu Esophagus. (G, H) RFC4 and GMPS mRNA levels were detected by RT-qPCR in 46 pairs of ESCC and adjacent normal tissues. (I) Protein levels in the three pairs of tissues. *p < 0.05, **p < 0.01, ***p < 0.001.
Therefore, we measured the expression levels of RFC4 and GMPS in 46 pairs of ESCC tumor samples and adjacent normal tissues to prove RFC4 and GMPS were increased in ESCC. Compared to the paired normal tissues, the results showed that RFC4 and GMPS were upregulated in the ESCC tumor tissues (Figure 5G, 5H). The protein levels of the two pairs of tissues were also significantly increased among the three pairs of tissues (Figure 5I).
RFC4 and GMPS were upregulated in the early stage of esophageal carcinoma and may be biomarkers for the early diagnosis of esophageal carcinoma
To found more evidences of RFC4 and GMPS participate in ESCC, the further analysis of UALCAN revealed that RFC4 and GMPS were significantly elevated in stage 1 esophageal carcinoma (Figure 6A, 6C). We also found that RFC4 and GMPS were upregulated at N0 based on the nodal metastasis status analyzed (Figure 6B, 6D). This funding may indicate that the expression of RFC4 and GMPS has increased, but metastasis did not occur yet or may be in the early stages. This funding was meaningful. Furthermore, combined with the ROC curves of 173 patients with ESCA and 46 pairs of ESCC samples (Figure 6E, 6F), we preliminarily concluded that RFC4 and GMPS are significant for identifying esophageal carcinoma in the early stage. In summary, we believe RFC4 and GMPS can be biomarkers for esophageal carcinoma identification and early diagnosis.
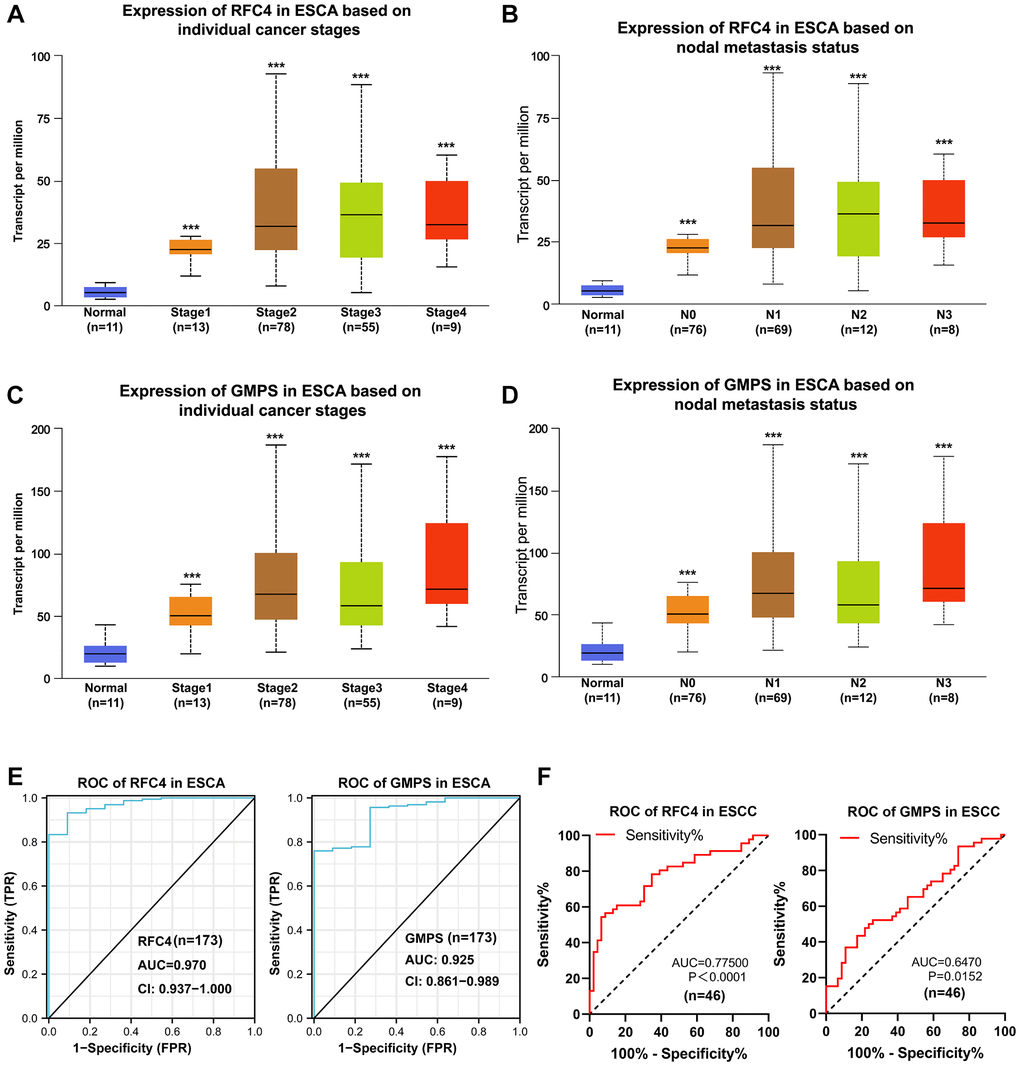
Figure 6. Upregulated RFC4 and GMPS are associated with the early diagnosis of esophageal carcinoma and may be the biomarkers for the early diagnosis of esophageal carcinoma. (A, B) Expression of RFC4 in patients with esophageal carcinoma based on the stage and nodal metastasis. (C, D) Expression of GMPS in patients with esophageal carcinoma based on the stage and nodal metastasis. (E) Receiver operating characteristic (ROC) curve analysis of RFC4 and GMPS in esophageal carcinoma (n = 173). (F) Receiver operating characteristic (ROC) curve analysis of RFC4 and GMPS in ESCC (n = 46). *p < 0.05, **p < 0.01, ***p < 0.001.
Moreover, to further investigate the association between the expression levels of RFC4 and GMPS and the clinicopathological features in ESCC, we divided the 46 tumor samples into two groups according to the cutoff values (median of RFC4, mean of GMPS) of RFC4 and GMPS mRNA expression levels. Next, we explored the correlation between RFC4 and GMPS expression and the clinicopathological parameters of patients with ESCC. A chi-square test was performed for the statistical analysis. Associations were observed between GMPS expression and vascular invasion (p = 0.017, Table 2). However, we did not find an association with RFC4 (Supplementary Table 2), but we did not have enough samples.
Table 2. Relationship between GMPS expression and clinicopathological features in ESCC.
| Clinical factor | Cases (n = 46) | GMPS expression | χ2 | p-value |
| Low (n = 24) | High (n = 22) |
| Gender |
| Male | 32 | 15 | 17 | 0.030 | 0.863 |
| Female | 14 | 9 | 5 | | |
| Age (years) |
| <61 | 19 | 10 | 9 | 0.003 | 0.958 |
| ≥61 | 27 | 14 | 13 | | |
| BMI |
| 18.5–23.9 | 35 | 17 | 18 | 0.761 | 0.383 |
| <18.5 OR ≥24 | 11 | 7 | 4 | | |
| Smoking status |
| Yes | 18 | 11 | 7 | 0.947 | 0.331 |
| No | 28 | 13 | 15 | | |
| Differentiation |
| Well (G1) | 4 | 2 | 2 | 0.170 | 1.000 |
| Moderate (G2) | 21 | 11 | 10 | | |
| Poor (G3) | 21 | 11 | 10 | | |
| pT status |
| Tis-2 | 12 | 5 | 7 | 0.947 | 0.331 |
| T3-4 | 34 | 19 | 15 | | |
| pN status |
| N0 | 32 | 16 | 16 | 0.199 | 0.665 |
| N1-3 | 14 | 8 | 6 | | |
| Pathological stage |
| 0 + I + II | 30 | 15 | 15 | 0.163 | 0.686 |
| III + IV | 16 | 9 | 7 | | |
| Vascular invasion |
| Yes | 5 | 1 | 4 | 5.690 | 0.017* |
| No | 41 | 23 | 18 | | |
Upregulated RFC4 and GMPS levels may be mediated by increased DNA copy number in ESCC
To further explore the reason for the increased RFC4 and GMPS in ESCC, we first explored genetic alterations in RFC4 and GMPS in esophageal carcinoma by a cBioPortal analysis. The results showed that amplification occurred in RFC4 and GMPS (RFC4, 40 cases (21.7%); GMPS, 33 cases (17.9%)) in 184 patients with esophageal cancer (Figure 7A). Further analysis found that gain and amplification were the most common copy number variations in esophageal carcinoma, especially ESCC (Figure 7B, 7D). Furthermore, for either RFC4 or GMPS, gain and amplification appeared in T1, N0 and M0. Therefore, we inferred that DNA copy number alterations mediated the increase in RFC4 and GMPS in the early stage of esophageal carcinoma (Figure 7C, 7E).
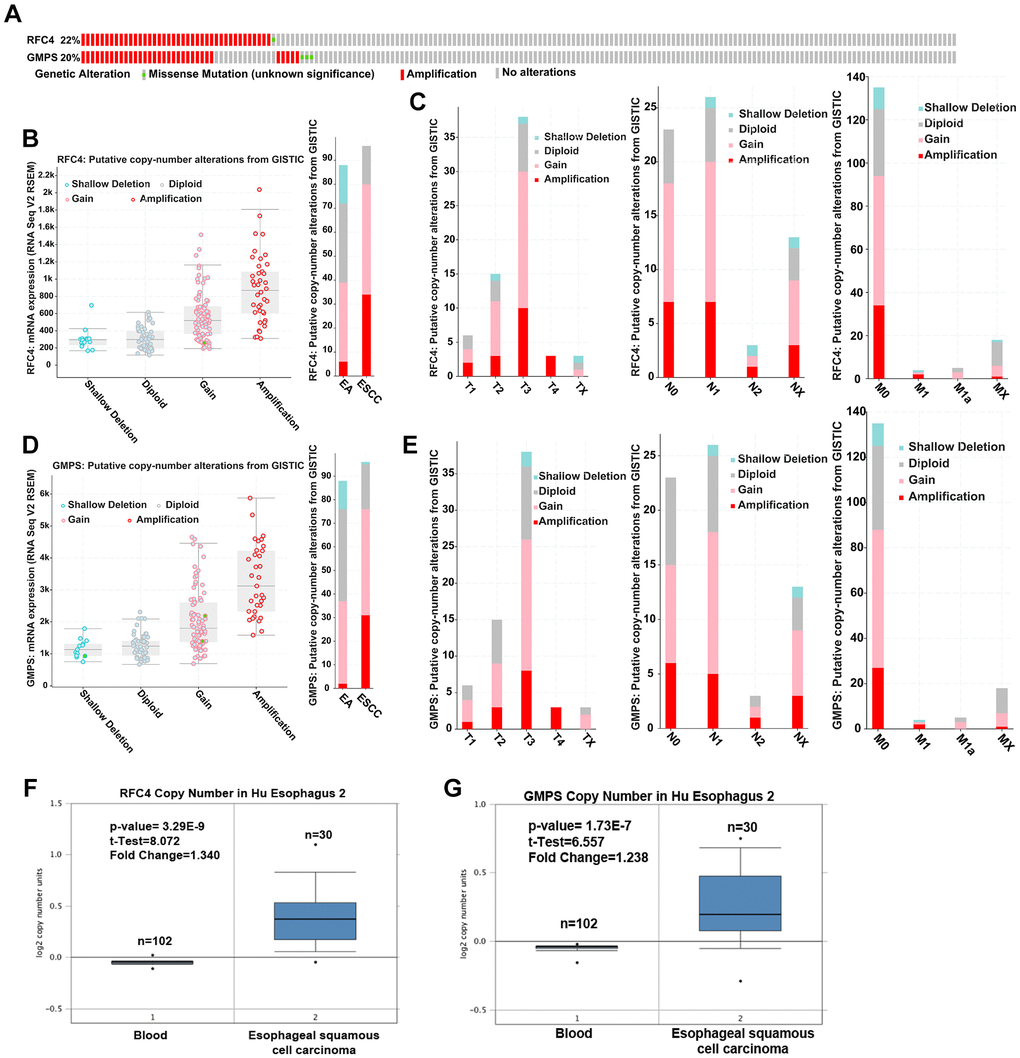
Figure 7. Upregulated RFC4 and GMPS levels may be mediated by DNA copy number alterations in ESCC. (A) Genetic alteration analysis of RFC4 and GMPS by cBioPortal. (B, D) Putative copy number alterations of RFC4 and GMPS in esophageal cancer. (C, E) Putative copy number alteration analysis of RFC4 and GMPS based on different T, N, and M stages. (F, G) DNA copy number of RFC4 and GMPS in Hu Esophagus 2. *p < 0.05, **p < 0.01, ***p < 0.001.
In addition, the DNA copy number was significantly increased in the ESCC patients based on an Oncomine online analysis (Figure 7F, 7G).
Some studies have reported that copy number changes may lead to cancer [19] and are associated with cancer patient prognosis [20]. Therefore, we inferred that the levels of RFC4 and GMPS may be mediated by an increased DNA copy number and related to the occurrence of esophageal cancer.
Exploration of the mechanisms of RFC4 and GMPS by GSEA and correlation analysis
To explore the potential molecular mechanisms of RFC4 and GMPS in esophageal carcinoma, GSEA from LinkedOmics was used. The GSEA results indicated that the top 4 pathways of the high expression in the RFC4 group were the cell cycle, spliceosome, DNA replication and RNA transport pathways (Figure 8A). In the high GMPS group, the top 4 pathways were the cell cycle, RNA transport, DNA replication and spliceosome pathways (Figure 8B). Surprisingly, we found that the functional enrichment of the two genes highly overlapped. Therefore, we subsequently explored the relationship between RFC4 and GMPS. The results indicated that the expression of RFC4 was highly correlated with the expression of GMPS in esophageal carcinoma in TCGA data (Figure 8C–8E). This correlation was also confirmed in our 46 pairs of samples (Figure 8F). Based on these findings, we hypothesized that there might be a synergistic relationship between RFC4 and GMPS.
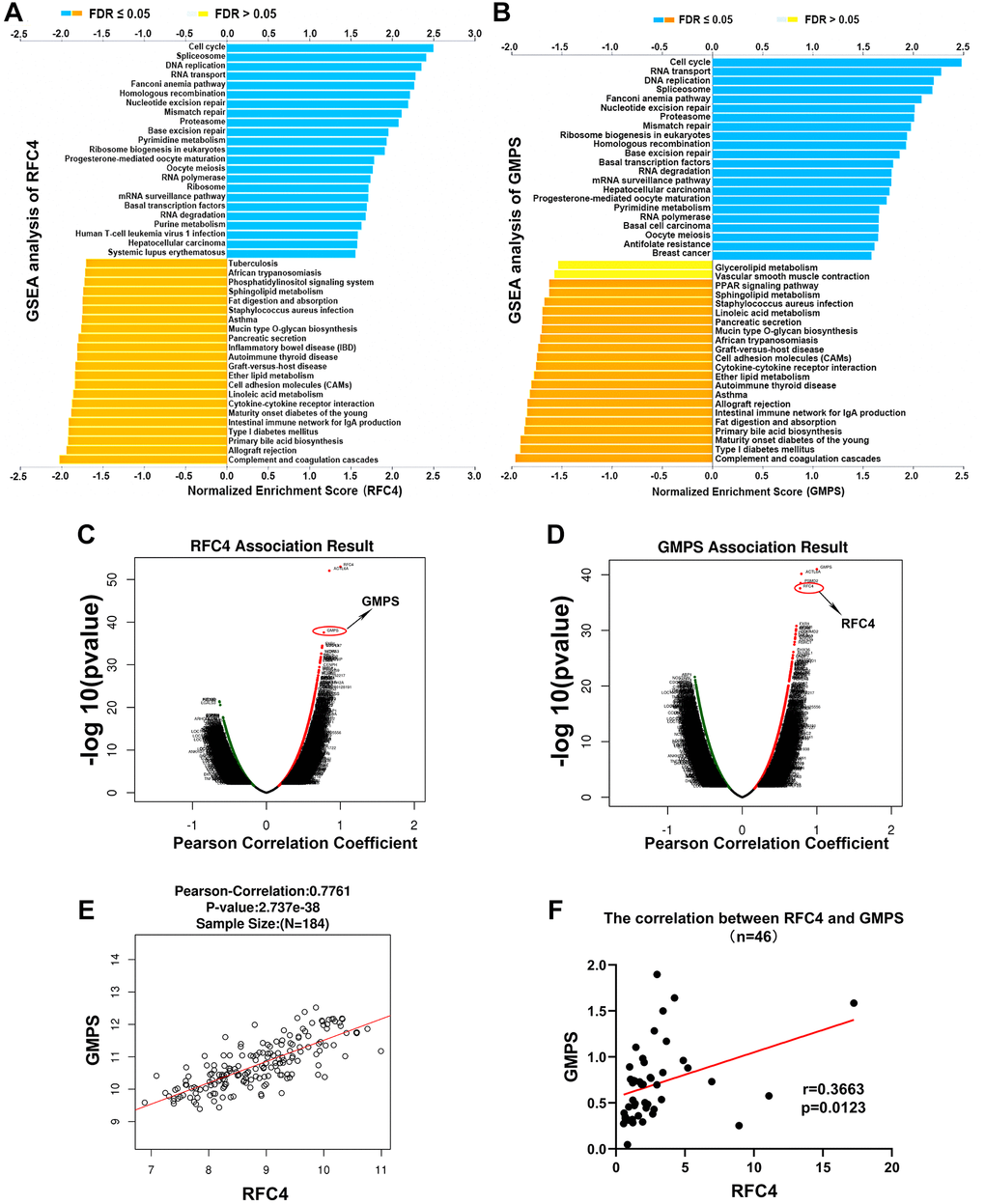
Figure 8. Exploration of the mechanism of RFC4 and GMPS based on GSEA and correlation analysis. (A, B) KEGG pathway analysis of RFC4 and GMPS based on GSEA. (C, D) Correlation coefficient analysis of RFC4 and GMPS in esophageal carcinoma. (E) Correlation between RFC4 and GMPS in TCGA. (F) Correlation between RFC4 and GMPS in 46 tumor samples. *p < 0.05, **p < 0.01, ***p < 0.001.
RFC4 and GMPS expression is correlated with tumor-infiltrating immune cells and immune escape in esophageal carcinoma
In recent years, tumor-infiltrating immune cells have been shown to participate in tumor growth and tumor development [21, 22]. Therefore, we investigated the relationship between RFC4 and GMPS expression and immune infiltration in esophageal carcinoma using the online tool TIMER. Six immune cell types and the tumor purity were assessed by TIMER. The results showed a significant correlation between RFC4 expression and tumor purity and dendritic cells (Figure 9A). And the GMPS expression was related to tumor purity, CD4+ T cells and neutrophils (Figure 9B). Further analysis revealed that a high expression of RFC4 also indicates poor prognosis, even when accompanied by high levels of tumor-infiltrating immune cells, including CD4+ T cells, CD8+ T cells, B cells, dendritic cells and monocytes (Figure 9C). When RFC4 was expressed at low levels, the high expression of B cells was associated with a better prognosis (Figure 9C). Similarly, a high GMPS expression accompanied by high levels of CD4+ T cells, CD 8+ T cells, B cells, dendritic cells and monocytes also indicated a poor prognosis (Figure 9D). A low expression of GMPS with a high expression of CD4+ T cells and dendritic cells showed a good prognosis (Figure 9D). In summary, we speculate that RFC4 and GMPS overexpression might influence tumor immune responses in the tumor microenvironment and that they may play a crucial role in esophageal carcinoma progression.
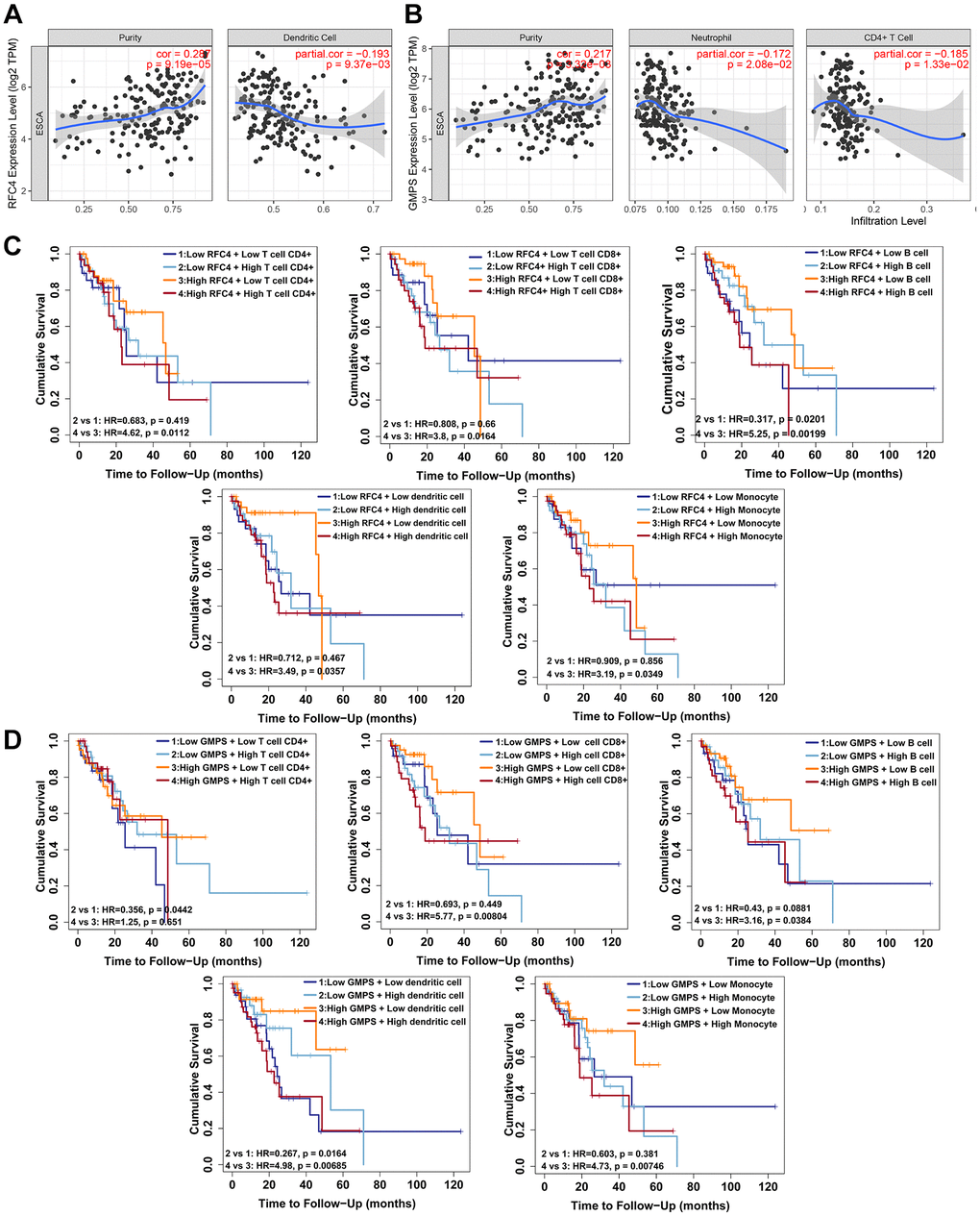
Figure 9. RFC4 and GMPS expression correlates with tumor-infiltrating immune cells and immune escape in esophageal carcinoma. (A) RFC4 expression was positively correlated with the tumor purity (r = 0.287, p < 0.05) but negatively correlated with Dendritic cells (r = −0.193, p < 0.05). (B) GMPS expression was positively correlated with the tumor purity (r = 0.217, p < 0.05) but negatively correlated with CD4+ T cells (r = −0.185, p < 0.05) and neutrophils (r =−0.172, p < 0.05). (C) A high level of RFC4 accompanied by a high expression of CD4+ T cells (p = 0.0112), CD 8+ T cells (p = 0.0164), B cells (p = 0.00199), dendritic cells (p = 0.0357) and monocytes (p = 0.0349) indicated a poor prognosis. When RFC4 was expressed at low levels, a high expression of B cells (p = 0.0201) was associated with a better prognosis. (D) A high level of GMPS accompanied by a high expression of CD 8+ T cells (p = 0.00804), B cells (p = 0.0384), dendritic cells (p = 0.00685) and monocytes (p = 0.00746) indicated a poor prognosis. When GMPS was expressed at low levels, a high expression of CD 8+ T cells (p = 0.0442) and dendritic cells (p = 0.0164) was associated with a better prognosis. p < 0.05 was considered significant.
Discussion
Esophageal carcinoma, especially ESCC, is a common health issue worldwide and usually has high mortality due to a late diagnosis and lack of efficient treatments [23, 24]. Usually, because the symptoms of the tumor are not specific during the early stages, endoscopy is used to screen early esophageal cancer [25, 26]. However, endoscopy has many limits. Endoscopy is expensive and not sufficiently available in many high-risk regions [23]. Therefore, a cheap, effective and acceptive diagnostic method is needed for early ESCC diagnosis.
In the present study, 3 datasets were analyzed to obtain DEGs between ESCC tissues and normal tissues. In total 64 DEGs were identified, including 22 downregulated genes and 42 upregulated genes. To gain more insight into ESCC, we found that RFC4 and GMPS in ESCC have rarely been investigated, and further studies are necessary.
RFC4 is a known subunit of the replication factor C complex, which functions mainly in DNA replication [27]. Many reports have shown that RFC4 may play an important role in the proliferation, progression, invasion, and metastasis of cancer cells [28]. Some findings suggest that RFC4 may be a potential prognostic biomarker and therapeutic target. For example, in colorectal cancer, RFC4 was correlated with tumor progression and predicted prognosis [29]. In another study, RFC4 decreased the growth and increased the chemosensitivity of hepatocellular carcinoma cells [30]. He et al. found that RFC4 was associated with significant survival in cervical squamous carcinoma [31]. In addition, RFC4 can act as a radio resistance factor in colorectal cancer [32]. However, few studies investigated ESCC. In our study, we found that RFC4 was increased in the early stage of esophageal carcinoma. By conducting a deep analysis, we inferred that DNA copy number alterations may mediate the elevation in genes.
GMPS catalyzes the final step in the de novo synthesis of guanine monophosphate [33]. It has been reported that GMPS plays a key role in cell proliferation and DNA replication. An early study also speculated that GMPS was a potential target for immunosuppressive therapy [34]. Recently, Zhang et al. found that TRIM21–SERPINB5 inhibits GMPS to protect nasopharyngeal carcinoma cells from radiation-induced apoptosis [35]. Wang et al. discovered that the inhibition of GMPS blocks prostate cancer growth [36]. Interestingly, our study is the first to report that GMPS is associated with ESCC. Similar to RFC4, GMPS was also increased in ESCC. In addition, the amplification of GMPS was also enhanced.
Altogether, we concluded that the increase in RCF4 and GMPS may be mediated by DNA copy number alterations, and that the increased expression of RFC4 and GMPS was associated with an early tumor stage and early nodal metastatic status in esophageal carcinoma. We inferred that RFC4 and GMPS can be biomarkers for esophageal carcinoma identification and early diagnosis. It was meaningful for early detection and diagnosis of esophageal cancer, and may improve the survival of patients with esophageal carcinoma. In addition, the GSEA showed that RFC4 and GMPS were significantly enriched in cell cycle, spliceosome, DNA replication and RNA transport. The function of RFC4 and GMPS in esophageal cancer were highly consistent. Through a correlation analysis, RFC4 was found to be strongly correlated with GMPS. We strongly considered that a synergistic relationship may exist between RFC4 and GMPS.
In recent years, immunotherapy has provided new hope for patients with cancers [37, 38]. Tumor-infiltrating immune cells are important for effective antitumor immunity [39, 40]. An early study showed that a high degree of CD8+ and CD4+ T-cell infiltration in ESCC was correlated with favorable clinical outcomes [41]. NK cells also play a vital role in ESCC [42]. In addition, our study explored the relationship between tumor-infiltrating immune cells and esophageal cancer. We uncovered that a high expression of RFC4 and GMPS accompanied by high levels of tumor-infiltrating immune cells was associated with a poor prognosis. However, a low expression of RFC4 and GMPS with a high expression of some tumor-infiltrating immune cells showed a good prognosis. Therefore, we reasonably concluded that RFC4 and GMPS involved in the immune regulation of esophageal cancer. A high expression of RFC4 and GMPS could mediate immune escape from esophageal cancer. In future, RFC4 and GMPS may serve as the targets for immunotherapy of esophageal cancer and improve the treatment of esophageal cancer.
However, the present study also has certain limitations, and further experiments need to be performed. Batch errors between many datasets cannot be avoided during analyses.
In conclusion, based on three datasets, we identified RFC4 and GMPS, which were upregulated in ESCC. Further analysis preliminarily revealed that RFC4 and GMPS are significant in the early stage and metastases and are possibly mediated by DNA copy number alterations. Additionally, GMPS was associated with vascular invasion in 46 tumor samples based on a clinical data analysis. In addition, RFC4 and GMPS perform the similar functions, and the expression of RFC4 was highly correlated with the expression of GMPS in esophageal cancer. Through a tumor-infiltrating immune analysis, we found that RFC4 and GMPS were correlated with some tumor-infiltrating immune cells and that an increased expression of RFC4 and GMPS could result in a poor prognosis. Finally, we concluded that RFC4 and GMPS are significant for the early diagnosis of esophageal carcinoma and that they may participate in the tumor immune response. Although the further experimental studies based on our findings are necessary, our findings are significant for the early diagnosis and treatment of ESCC.
Materials and Methods
Data collection and identification of DEGs
GSE20347 [43] and GSE17351 [44] from GEO and our private sequencing data of ESCC were selected for our study. For the GEO datasets, the selection criteria were as follows: 1) tissues are diagnosed with ESCC and have matched normal tissues; and 2) probes can be converted into gene symbols and include complete information for the analysis. Finally, the three datasets contained 25 ESCC samples and 25 matched normal tissues.
The GEO2R tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/) was applied to screen the DEGs between the tumor and nontumor samples. The DEGs were screened by |log2FC| > 1 and an adjusted p-value < 0.05. Then, online Venn diagram software (http://bioinformatics.psb.ugent.be/webtools/Venn/) was used for visualization of the data.
PPI network construction and enrichment analysis of DEGs
First, we used the STRING (https://string-db.org/) database to construct protein-protein interaction (PPI) networks of the DEGs in ESCC. Then, we used Cytoscape software [45] to visualize the PPI networks.
The R package “clusterProfiler” was used for the GO enrichment and KEGG pathway analyses of the DEGs. P < 0.05 indicated statistical significance.
Hub gene selection and analysis
The cytoHubba module of Cytoscape was used to screen hub genes based on a degree >4. Then, functional and pathway enrichment analyses of the hub genes were performed with the R package “clusterProfiler”.
Expression analysis of RFC4 and GMPS in public datasets
The expression of RFC4 and GMPS in cancers was examined in the TIMER database [46, 47]. The mRNA expression and DNA copy number of RFC4 and GMPS in ESCC were further analyzed by using the Oncomine database (https://www.oncomine.org/resource/main.html). The relationship between the mRNA levels of RFC4/GMPS and the pathological features of patients with esophageal carcinoma in terms of the cancer stage and nodal metastasis was analyzed using UALCAN [48].
Genetic alteration analysis using cBioPortal
cBioPortal (https://www.cbioportal.org/) is an open-access resource for multidimensional cancer genomic data [49, 50]. We used cBioPortal to explore genetic alterations in RFC4 and GMPS.
Gene set enrichment analysis (GSEA) and correlation analysis using LinkedOmics
The GSEA of 184 esophageal carcinoma samples from TCGA was performed using LinkedOmics (http://www.linkedomics.org/admin.php datasets. The relationship of RFC4 and GMPS in ESCC was also analyzed by using LinkedOmics.
Tumor-infiltrating immune cell (TILs) analysis
The online tool TIMER (https://cistrome.shinyapps.io/timer/) [46, 47] was used to investigate the correlation between RFC4/GMPS and the tumor purity and infiltrating immune cells (B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and dendritic cells). TIMER2.0 (http://timer.cistrome.org/) [51] was used for the survival analysis of RFC4 and GMPS with the tumor immune cell infiltration.
Clinical samples
Forty-six pairs of fresh ESCC and adjacent normal tissues were collected from the Department of Thoracic Surgery at the First Affiliated Hospital of Sun Yat-Sen University (FAHSYSU). All tissue samples were directly frozen, and RNA was extracted in a timely manner. All tissue samples were endorsed by the Medical Ethical Committee of the SYSUCC and the FAHSYSU, and written consent documents were obtained from all patients.
RNA extraction and quantitative real-time PCR analysis (qRT-PCR)
The total RNA was isolated by TRIzol reagent (Invitrogen). Reverse transcription and qRT-PCR were carried out using SYBR Green Master Mix (YEASEN) according to the manufacturer’s protocol. GAPDH was used as an internal control. The sequences of the primers were as follows:
GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′;
GAPDH reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′;
RFC4 forward, 5′-GGCAGCTTTAAGACGTACCATGG-3′;
RFC4 reverse, 5′-TCTGACAGAGGCTTGAAGCGGA-3′;
GMPS forward, 5′-CCCATCACAATGACACAGAGCTC-3′;
GMPS reverse, 5′-CTGGAAGTCCAAGTTCTCTGCC-3′.
The relative expression was calculated using the 2−ΔΔCt method.
Western blotting analysis
A Western blot analysis was performed according to standard methods [52]. A BCA Protein Quantification Kit (YEASEN) was used to measure the concentration. The antibodies against RFC4 and GMPS were purchased from Proteintech. GAPDH was used as an internal control.
Statistical analysis
The data were analyzed using GraphPad Prism 8.0. The expression levels of the DEGs between the tumor and adjacent normal tissues were compared by paired two- tailed t-test. A chi-square test was performed to evaluate the relationship between the clinicopathological features and the expression levels of RFC4 and GMPS. A p-value <0.05 was considered statistically significant.
Abbreviations
EA: esophageal adenocarcinoma;
ESCC: esophageal squamous cell carcinoma;
DEGs: differentially expressed genes;
RFC4: replication factor C subunit 4;
GMPS: guanine monophosphate synthase;
GEO: The Gene Expression Omnibus database;
TCGA: The Cancer Genome Atlas;
GO: Gene ontology;
KEGG: Kyoto Encyclopedia of Genes and Genomes;
PPI: protein-protein interaction;
GSEA: Gene set enrichment analysis;
TILs: Tumor-infiltrating immune cell.
Author Contributions
Jing Wang: Analysis and interpretation of data, writing and editing. Fei-Fei Luo: Analysis, writing and editing. Tie-Jun Huang: Data curation and visualization. Yan Mei: Data curation. Li-Xia Peng: Technical support. Chao-Nan Qian: Funding acquisition. Bi-Jun Huang: Funding acquisition and study supervision.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81972785, No. 81773162, No. 81572901 to B.H., No. 82073220, No. 81872384 and No. 81672872 to C.Q.), the Provincial Natural Science Foundation of Guangdong, China (No. 2017A030313866 to B.H., No. 2016A030311011 to C.Q.).
References
-
1.
Hesari A, Azizian M, Sheikhi A, Nesaei A, Sanaei S, Mahinparvar N, Derakhshani M, Hedayt P, Ghasemi F, Mirzaei H. Chemopreventive and therapeutic potential of curcumin in esophageal cancer: Current and future status. Int J Cancer. 2019; 144:1215–26. https://doi.org/10.1002/ijc.31947 [PubMed]
-
2.
Murphy G, McCormack V, Abedi-Ardekani B, Arnold M, Camargo MC, Dar NA, Dawsey SM, Etemadi A, Fitzgerald RC, Fleischer DE, Freedman ND, Goldstein AM, Gopal S, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017; 28:2086–93. https://doi.org/10.1093/annonc/mdx279 [PubMed]
-
3.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. https://doi.org/10.3322/caac.21492 [PubMed]
-
4.
Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019; 17:855–83. https://doi.org/10.6004/jnccn.2019.0033 [PubMed]
-
5.
Kim JA, Shah PM. Screening and prevention strategies and endoscopic management of early esophageal cancer. Chin Clin Oncol. 2017; 6:50. https://doi.org/10.21037/cco.2017.09.05 [PubMed]
-
6.
Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003; 349:2241–52. https://doi.org/10.1056/NEJMra035010 [PubMed]
-
7.
Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015; 21:7933–43. https://doi.org/10.3748/wjg.v21.i26.7933 [PubMed]
-
8.
Visser E, Markar SR, Ruurda JP, Hanna GB, van Hillegersberg R. Prognostic Value of Lymph Node Yield on Overall Survival in Esophageal Cancer Patients: A Systematic Review and Meta-analysis. Ann Surg. 2019; 269:261–68. https://doi.org/10.1097/SLA.0000000000002824 [PubMed]
-
9.
Kiyozumi Y, Baba Y, Okadome K, Yagi T, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Komohara Y, Baba H. IDO1 Expression Is Associated With Immune Tolerance and Poor Prognosis in Patients With Surgically Resected Esophageal Cancer. Ann Surg. 2019; 269:1101–08. https://doi.org/10.1097/SLA.0000000000002754 [PubMed]
-
10.
Siersema PD. Esophageal Cancer Awareness Issue 2019. Endoscopy. 2019; 51:291–92. https://doi.org/10.1055/a-0858-6770 [PubMed]
-
11.
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013; 381:400–12. https://doi.org/10.1016/S0140-6736(12)60643-6 [PubMed]
-
12.
Alsop BR, Sharma P. Esophageal Cancer. Gastroenterol Clin North Am. 2016; 45:399–412. https://doi.org/10.1016/j.gtc.2016.04.001 [PubMed]
-
13.
Meves V, Behrens A, Pohl J. Diagnostics and Early Diagnosis of Esophageal Cancer. Viszeralmedizin. 2015; 31:315–18. https://doi.org/10.1159/000439473 [PubMed]
-
14.
Oliver GR, Hart SN, Klee EW. Bioinformatics for clinical next generation sequencing. Clin Chem. 2015; 61:124–35. https://doi.org/10.1373/clinchem.2014.224360 [PubMed]
-
15.
Wang K, Chen R, Feng Z, Zhu YM, Sun XX, Huang W, Chen ZN. Identification of differentially expressed genes in non-small cell lung cancer. Aging (Albany NY). 2019; 11:11170–85. https://doi.org/10.18632/aging.102521 [PubMed]
-
16.
Meng LB, Shan MJ, Qiu Y, Qi R, Yu ZM, Guo P, Di CY, Gong T. TPM2 as a potential predictive biomarker for atherosclerosis. Aging (Albany NY). 2019; 11:6960–82. https://doi.org/10.18632/aging.102231 [PubMed]
-
17.
Su H, Hu N, Yang HH, Wang C, Takikita M, Wang QH, Giffen C, Clifford R, Hewitt SM, Shou JZ, Goldstein AM, Lee MP, Taylor PR. Global gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypes. Clin Cancer Res. 2011; 17:2955–66. https://doi.org/10.1158/1078-0432.CCR-10-2724 [PubMed]
-
18.
Hu N, Clifford RJ, Yang HH, Wang C, Goldstein AM, Ding T, Taylor PR, Lee MP. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics. 2010; 11:576. https://doi.org/10.1186/1471-2164-11-576 [PubMed]
-
19.
Kachouie NN, Deebani W, Christiani DC. Identifying Similarities and Disparities Between DNA Copy Number Changes in Cancer and Matched Blood Samples. Cancer Invest. 2019; 37:535–45. https://doi.org/10.1080/07357907.2019.1667368 [PubMed]
-
20.
Smith JC, Sheltzer JM. Systematic identification of mutations and copy number alterations associated with cancer patient prognosis. Elife. 2018; 7:e39217. https://doi.org/10.7554/eLife.39217 [PubMed]
-
21.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011; 331:1565–70. https://doi.org/10.1126/science.1203486 [PubMed]
-
22.
Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. 2019; 16:6–18. https://doi.org/10.1038/s41423-018-0027-x [PubMed]
-
23.
Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D, Iyer PG. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018; 88:413–26. https://doi.org/10.1016/j.gie.2018.04.2352 [PubMed]
-
24.
Talukdar FR, di Pietro M, Secrier M, Moehler M, Goepfert K, Lima SSC, Pinto LFR, Hendricks D, Parker MI, Herceg Z. Molecular landscape of esophageal cancer: implications for early detection and personalized therapy. Ann N Y Acad Sci. 2018; 1434:342–59. https://doi.org/10.1111/nyas.13876 [PubMed]
-
25.
di Pietro M, Canto MI, Fitzgerald RC. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology. 2018; 154:421–36. https://doi.org/10.1053/j.gastro.2017.07.041 [PubMed]
-
26.
Mönig S, Chevallay M, Niclauss N, Zilli T, Fang W, Bansal A, Hoeppner J. Early esophageal cancer: the significance of surgery, endoscopy, and chemoradiation. Ann N Y Acad Sci. 2018; 1434:115–23. https://doi.org/10.1111/nyas.13955 [PubMed]
-
27.
Liu L, Tao T, Liu S, Yang X, Chen X, Liang J, Hong R, Wang W, Yang Y, Li X, Zhang Y, Li Q, Liang S, et al. An RFC4/Notch1 signaling feedback loop promotes NSCLC metastasis and stemness. Nat Commun. 2021; 12:2693. https://doi.org/10.1038/s41467-021-22971-x [PubMed]
-
28.
Li Y, Gan S, Ren L, Yuan L, Liu J, Wang W, Wang X, Zhang Y, Jiang J, Zhang F, Qi X. Multifaceted regulation and functions of replication factor C family in human cancers. Am J Cancer Res. 2018; 8:1343–55. [PubMed]
-
29.
Xiang J, Fang L, Luo Y, Yang Z, Liao Y, Cui J, Huang M, Yang Z, Huang Y, Fan X, Wang H, Wang L, Peng J, Wang J. Levels of human replication factor C4, a clamp loader, correlate with tumor progression and predict the prognosis for colorectal cancer. J Transl Med. 2014; 12:320. https://doi.org/10.1186/s12967-014-0320-0 [PubMed]
-
30.
Arai M, Kondoh N, Imazeki N, Hada A, Hatsuse K, Matsubara O, Yamamoto M. The knockdown of endogenous replication factor C4 decreases the growth and enhances the chemosensitivity of hepatocellular carcinoma cells. Liver Int. 2009; 29:55–62. https://doi.org/10.1111/j.1478-3231.2008.01792.x [PubMed]
-
31.
He Y, Hu S, Zhong J, Cheng A, Shan N. Identification of significant genes signatures and prognostic biomarkers in cervical squamous carcinoma via bioinformatic data. PeerJ. 2020; 8:e10386. https://doi.org/10.7717/peerj.10386 [PubMed]
-
32.
Wang XC, Yue X, Zhang RX, Liu TY, Pan ZZ, Yang MJ, Lu ZH, Wang ZY, Peng JH, Le LY, Wang GY, Peng QH, Meng Y, et al. Genome-wide RNAi Screening Identifies RFC4 as a Factor That Mediates Radioresistance in Colorectal Cancer by Facilitating Nonhomologous End Joining Repair. Clin Cancer Res. 2019; 25:4567–79. https://doi.org/10.1158/1078-0432.CCR-18-3735 [PubMed]
-
33.
Welin M, Lehtiö L, Johansson A, Flodin S, Nyman T, Trésaugues L, Hammarström M, Gräslund S, Nordlund P. Substrate specificity and oligomerization of human GMP synthetase. J Mol Biol. 2013; 425:4323–33. https://doi.org/10.1016/j.jmb.2013.06.032 [PubMed]
-
34.
Nakamura J, Lou L. Biochemical characterization of human GMP synthetase. J Biol Chem. 1995; 270:7347–53. https://doi.org/10.1074/jbc.270.13.7347 [PubMed]
-
35.
Zhang P, Li X, He Q, Zhang L, Song K, Yang X, He Q, Wang Y, Hong X, Ma J, Liu N. TRIM21-SERPINB5 aids GMPS repression to protect nasopharyngeal carcinoma cells from radiation-induced apoptosis. J Biomed Sci. 2020; 27:30. https://doi.org/10.1186/s12929-020-0625-7 [PubMed]
-
36.
Wang Q, Guan YF, Hancock SE, Wahi K, van Geldermalsen M, Zhang BK, Pang A, Nagarajah R, Mak B, Freidman N, Horvath LG, Turner N, Holst J. Inhibition of guanosine monophosphate synthetase (GMPS) blocks glutamine metabolism and prostate cancer growth. J Pathol. 2021; 254:135–46. https://doi.org/10.1002/path.5665 [PubMed]
-
37.
Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Baba H. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020; 111:3132–41. https://doi.org/10.1111/cas.14541 [PubMed]
-
38.
Yagi T, Baba Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. PD-L1 Expression, Tumor-infiltrating Lymphocytes, and Clinical Outcome in Patients With Surgically Resected Esophageal Cancer. Ann Surg. 2019; 269:471–78. https://doi.org/10.1097/SLA.0000000000002616 [PubMed]
-
39.
Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol. 2015; 194:2985–91. https://doi.org/10.4049/jimmunol.1403134 [PubMed]
-
40.
Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, Naveaux C, Lodewyckx JN, Boisson A, Duvillier H, Craciun L, Ameye L, Veys I, et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019; 5:e129641. https://doi.org/10.1172/jci.insight.129641 [PubMed]
-
41.
Jiang Y, Lo AWI, Wong A, Chen W, Wang Y, Lin L, Xu J. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017; 8:30175–89. https://doi.org/10.18632/oncotarget.15621 [PubMed]
-
42.
Strizova Z, Vachtenheim J Jr, Snajdauf M, Lischke R, Bartunkova J, Smrz D. Tumoral and paratumoral NK cells and CD8+ T cells of esophageal carcinoma patients express high levels of CD47. Sci Rep. 2020; 10:13936. https://doi.org/10.1038/s41598-020-70771-y [PubMed]
-
43.
Hu J, Li R, Miao H, Wen Z. Identification of key genes for esophageal squamous cell carcinoma via integrated bioinformatics analysis and experimental confirmation. J Thorac Dis. 2020; 12:3188–99. https://doi.org/10.21037/jtd.2020.01.33 [PubMed]
-
44.
Lee JJ, Natsuizaka M, Ohashi S, Wong GS, Takaoka M, Michaylira CZ, Budo D, Tobias JW, Kanai M, Shirakawa Y, Naomoto Y, Klein-Szanto AJ, Haase VH, Nakagawa H. Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis. 2010; 31:427–34. https://doi.org/10.1093/carcin/bgp326 [PubMed]
-
45.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13:2498–504. https://doi.org/10.1101/gr.1239303 [PubMed]
-
46.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017; 77:e108–10. https://doi.org/10.1158/0008-5472.CAN-17-0307 [PubMed]
-
47.
Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016; 17:174. https://doi.org/10.1186/s13059-016-1028-7 [PubMed]
-
48.
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BV, Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017; 19:649–58. https://doi.org/10.1016/j.neo.2017.05.002 [PubMed]
-
49.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013; 6:pl1. https://doi.org/10.1126/scisignal.2004088 [PubMed]
-
50.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012; 2:401–04. https://doi.org/10.1158/2159-8290.CD-12-0095 [PubMed]
-
51.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020; 48:W509–14. https://doi.org/10.1093/nar/gkaa407 [PubMed]
-
52.
Kurien BT, Scofield RH. Western blotting. Methods. 2006; 38:283–93. https://doi.org/10.1016/j.ymeth.2005.11.007 [PubMed]