ANT-DBS relieved apoptosis and protected neurons in epileptic monkeys
Hippocampal atrophy was obvious in the EP and EP-sham-DBS groups. Hippocampal atrophy, however, did not occur in ANT-DBS treated animals (Figure 2D). Numerous studies have revealed that hippocampal atrophy is mainly caused by neuronal loss, so we evaluated the protein level of NeuN, a specific neuron marker, and the level of cleaved-caspase-3 and cleaved-caspase-9, markers of apoptosis. Also, the caspase-3 and caspase-9 were evaluated. Compared with the control group, there was a significant decrease in NeuN (F(3,8)= 10.007, P <0.01) and an increase in cleaved-caspase-3 (F(3,8)= 20.735, P < 0.001) and cleaved-caspase-9 (F(3,8)= 6.148, P < 0.05) in the EP and EP-sham-DBS groups, indicating severe neuronal loss and apoptosis. This phenomenon was alleviated after ANT stimulation in the EP-DBS group, with enhanced NeuN, and reduced cleaved-caspase-3 and cleaved-caspase-9 level in comparison with the EP and EP-sham-DBS groups. Meantime, it is found that the decreased caspase-3 (F(3,8)= 10.704, P <0.01) and caspase-9 (F(3,8)= 20.096 P < 0.001) were reversed by ANT-DBS (Figure 2E–2J).
We also evaluated the cell injury and the mitochondrial injury follow the criteria using TEM (Tables 1 and 2). In the EP and EP-sham-DBS groups, cell injury was severe in the hippocampus with numerous fragments from disintegrated organelles. The neurons were predominantly pyknotic, with or without complete cell membranes. The neuronal nuclei were either deformed or ruptured, with extensive karyorrhexis and karyolysis. However, EP-DBS group animals had neuronal injury no different from control group, presenting cellular integrity with some swelling of organelles in some neurons (Figure 3A). According to the criteria, the injury scores were elevated in the EP and EP-sham-DBS groups compared with the control group, and were reduced in the EP-DBS group (F(3,8)= 20.837, P < 0.001) (Figure 3B).
Table 1. Scoring system for neuronal injury by transmission electron microscopy.
| Score | Injury observed in TEM |
| 0 | Basically normal. |
| 1 | Slightly injured, focally distended ER, condensed or swollen M, fovea on karyolemma. |
| 2 | Mildly injured, general swelling of organelles, clearly decreased cytoplasmic electron density, major depression of karyolemma. |
| 3 | Moderately injured, severe swelling of the entire cell, formation of cytoplasmic vacuoles or blebs, evident cell shrinkage, transparent cytoplasm. |
| 4 | Severely injured, pyknotic cells with deformed, but integrated cell membrane and karyolemma, apoptosis. |
| 5 | Near death, pyknotic cells with trace of karyorrhexis or karyolysis, disruption of cell membrane, rupture of karyolemma, disintegration of organelles, apoptotic bodies. |
| M: mitochondria. ER: endoplasmic reticulum. Minimal increment: 0.5 |
Table 2. Scoring system for mitochondrial injury by transmission electron micrography.
| Score | Observation |
| 0 | Normal mitochondria (mitochondria appeared highly dense with well-organized cristae) |
| 1 | Early swelling as manifested by early clearing of matrix density and separation of cristae (a large amorphous matrix density and a linear density are present) |
| 2 | More marked swelling as manifested by further clearing of matrix density and separation of cristae |
| 3 | More extensive mitochondrial swelling with disruption of cristae |
| 4 | Severe mitochondrial swelling with disruption of cristae and rupture of inner and outer mitochondrial membranes |
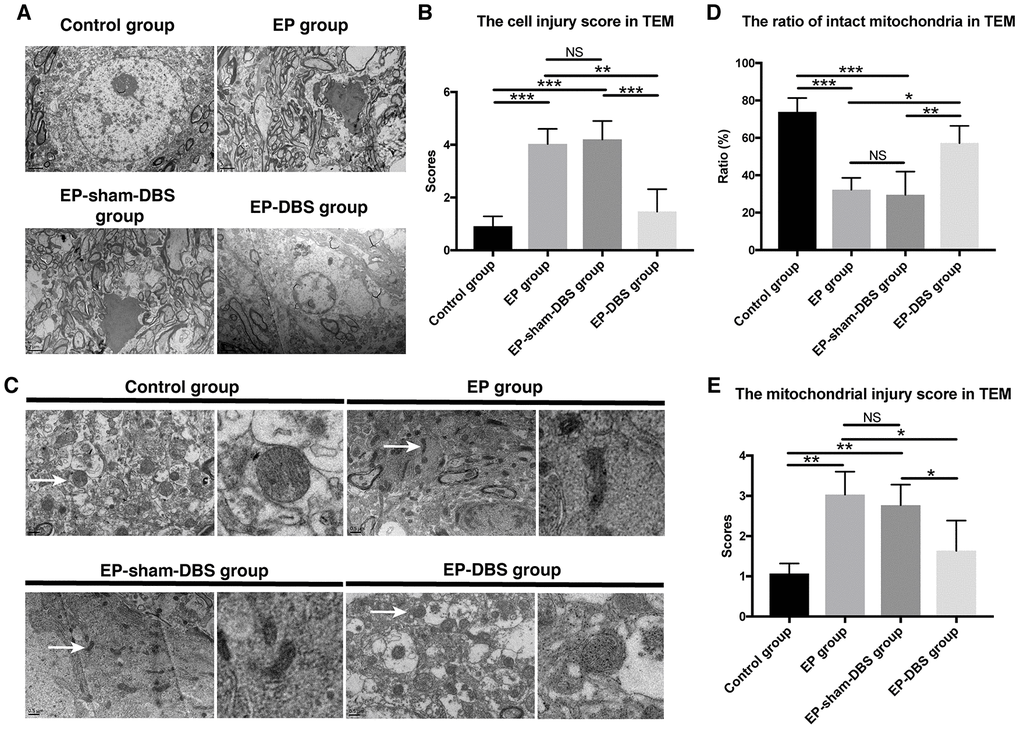
Figure 3. ANT-DBS protected hippocampal neurons of the epileptic monkeys. (A) Neuronal ultrastructure by TEM. The injury was severe in the hippocampus of EP and EP-sham-DBS groups. The injury, however, was relieved by ANT-DBS. Magnification: ×1000. (B) Higher injury grades were obtained in the EP and EP-sham-DBS groups, and this grade was alleviated by ANT-DBS. (n=3 in each group; in each monkey, twenty cells were randomly selected, and the average score for each monkey was recorded) (C) Morphology of mitochondria (white arrow) by TEM. Normal or slightly abnormal morphology of mitochondria was seen in the control and EP-DBS groups. The EP and EP-sham-DBS groups showed swelling of the mitochondrial matrix, sometimes with disrupted membrane integrity. Magnification: ×3000. The right column shows a closer view of mitochondria. (D) The number of intact mitochondria in the EP and EP-sham-DBS groups was lower than that in the control and EP-DBS groups. (n=3 in each group; in each monkey, twenty fields were randomly selected, and the average number for each monkey was recorded) (E) The grade of mitochondrial injury was higher in the EP and EP-sham-DBS groups, and reversed by ANT-DBS (n=3 in each group; in each monkey, twenty cells were randomly selected, and the average grade for each monkey was recorded). *P< 0.05; **P< 0.01; ***P< 0.001; NS, P> 0.05. Data were presented as mean ± SD.
Marked mitochondrial ultrastructural injury was observed in the EP and EP-sham-DBS groups, with significant swelling of the mitochondrial matrix. In some instances, mitochondrial swelling was accompanied by disruption of membrane integrity. In contrast, mitochondria in the EP-DBS group had normal morphology, with no or only slight evidence of swelling, outer membrane breakage, or intracristal dilation, which was similar to that in the control group (Figure 3C). According to the criteria, grades 0 and 1 were considered as intact mitochondria, and less intact mitochondria were found in the epileptic model, whereas, ratio of intact mitochondria was enhanced in monkeys that received ANT-DBS (F(3,8)= 16.116, P < 0.001) (Figure 3D). The mean mitochondrial injury grade was further evaluated in term of the criteria mentioned above, and showed a similar tendency (F(3,8)= 8.567, P < 0.01) (Figure 3E).
ANT-DBS inhibited BDNF–TrkB signaling, and subsequently reduced Akt and ERK phosphorylation in the hippocampus of epileptic monkeys
Immunofluorescence staining of BDNF and NeuN was carried to evaluate BDNF expression in neurons. Significantly more BDNF was observed in hippocampal CA1 (F(3,8)= 4.620, P < 0.05) and CA3 (F(3,8)= 13.564, P < 0.01) neurons in the EP and EP-sham-DBS groups, and less was detected in the EP-DBS group (Figure 4). BDNF–TrkB plays an essential role in regulating autophagy via the Akt and ERK pathway [21]. In the EP and EP-sham-DBS groups, BDNF (F(3,8)= 19.242, P < 0.001) and TrkB (F(3,8)= 7.107, P < 0.05) levels were elevated compared with the control group. With ANT-DBS, notably less BDNF and TrkB were detected (Figure 5A–5C).
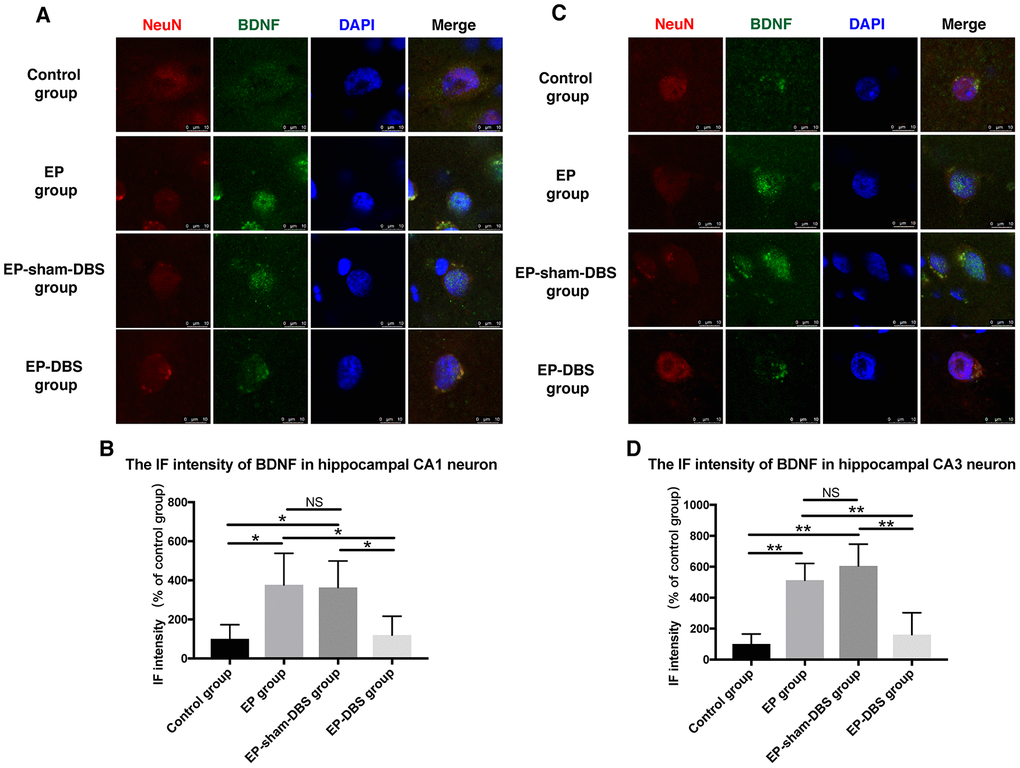
Figure 4. ANT-DBS reduced BDNF expression in the hippocampal neurons of epileptic monkeys. Immunofluorescence staining of BDNF and NeuN in hippocampal CA1 (A) and CA3 (C) neurons. NeuN is a specific neuronal marker. BDNF expression was elevated in EP and EP-sham-DBS groups in hippocampal CA1 and CA3 neurons and reduced by ANT-DBS. Immunofluorescent intensity of BDNF in hippocampal CA1 (B) and CA3 (D) neurons was quantified. (n=3 in each group; in each monkey, a total of thirty cells in CA1 or CA3, the average immunofluorescent intensity of these cells was recorded) *P < 0.05; **P < 0.01; NS, P > 0.05. Data were presented as mean ± SD.
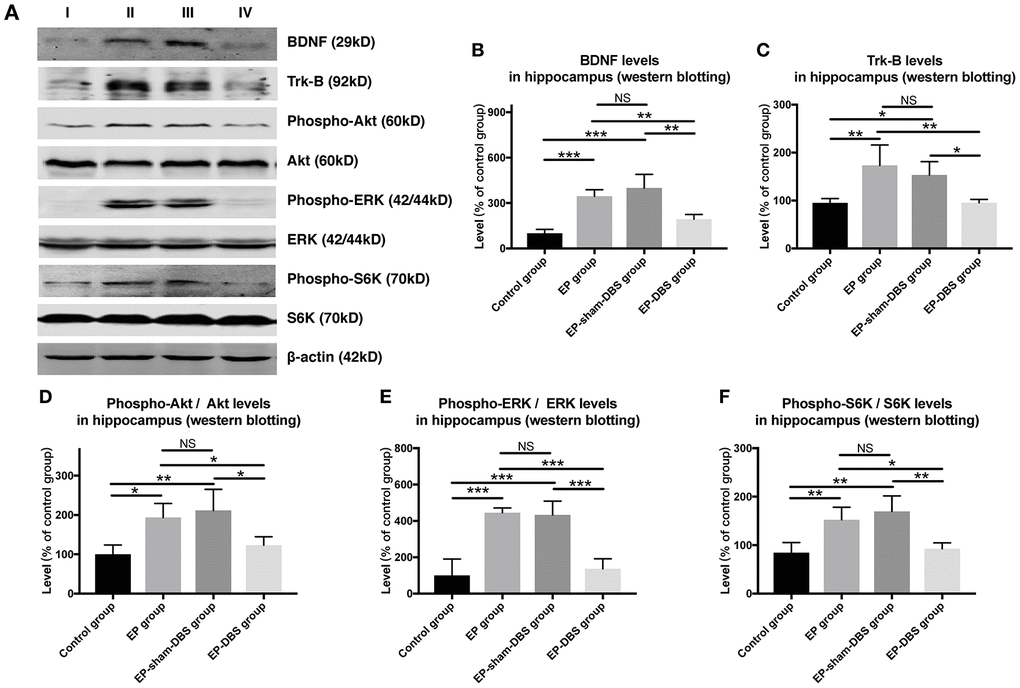
Figure 5. ANT-DBS inhibited BDNF-TrkB pathway and its downstream regulator in the hippocampus of epileptic monkeys. (A) Analysis of BDNF, TrkB, p-ERK, ERK, p-Akt, Akt, p-S6K and S6K by western blotting. (B–C) In EP and EP-sham-DBS groups, BDNF and TrkB levels were elevated compared with the control group. With ANT-DBS, less BDNF and TrkB were observed. (n=3 in each group) (D–F) In the epileptic animal model, the ratio of p-Akt/Akt, p-ERK/ERK and p-S6K/S6K was enhanced in the hippocampus, and reversed by ANT-DBS. (n=3 in each group) *P < 0.05; **P < 0.01; ***P < 0.001; NS, P > 0.05. Data were presented as mean ± SD. I, control group; II, EP group; III, EP-sham-DBS group; IV, EP-DBS group.
Akt and ERK are the most important downstream regulators of the BDNF–TrkB signaling pathway, and activation of the pathway is shown by phosphorylation of Akt and ERK (p-Akt and p-ERK). In the epileptic animal model, p-Akt (F(3,8)= 6.729, P < 0.05) and p-ERK (F(3,8)= 23.642, P < 0.001) were enhanced in the hippocampus; however, p-Akt and p-ERK levels were decreased by ANT-DBS, and showed a similar trend to that shown by BDNF–TrkB (Figure 5A, 5D and 5E).
Phosphorylation of S6K (p-S6K) has been used as a marker of activation by mTORC1 and is positively correlated with the inhibition of autophagy. We found that ANT-DBS reversed the increased p-S6K (F(3,8)= 9.635, P < 0.01) level in epileptic monkeys (Figure 5A and 5F). No significant difference in above was detected between the EP and EP-sham-DBS groups.
ANT-DBS activated autophagy in the hippocampus of epileptic monkeys
In the formation of autophagosomes, the cytosolic form of LC3I is converted to the lipid-conjugated LC3II form (a marker of autophagosome). To investigate the effect of ANT-DBS on autophagy induction, LC3II protein level was assessed. ANT-DBS resulted in up-regulation of LC3II compared to those in the EP and EP-sham-DBS groups (F(3,8)= 18.766, P < 0.001) (Figure 6A and 6B). P62 is a substrate of autophagy and its level was determined in the different groups. The elevation of P62 protein level in the EP and EP-sham-DBS groups was reduced by ANT-DBS (F(3,8)= 9.527, P < 0.01) (Figure 6A and 6C). These results indicated that autophagosome formation was induced by ANT-DBS in epileptic monkeys.
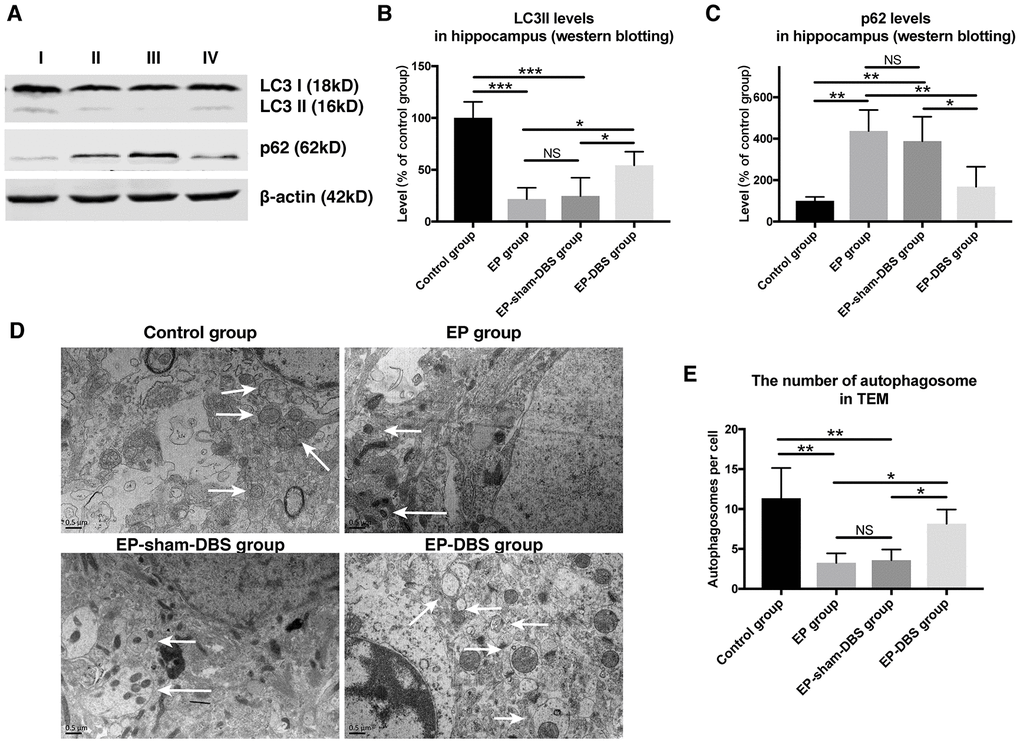
Figure 6. ANT-DBS activated autophagy in the hippocampus of epileptic monkeys. (A) Analysis of LC3II and p62 by western blotting. (B) Levels of LC3II were decreased in EP and EP-sham-DBS groups. ANT-DBS normalized LC3II level in the EP-DBS group. (n=3 in each group) (C) In the EP and EP-sham-DBS groups, p62 levels were increased, and decreased by ANT-DBS. (n=3 in each group) (D) Autophagosome observed by TEM. The left column shows the morphology of the autophagosome by TEM. In the other columns, the autophagosomes (white arrow) are shown around the nucleus. (E) The number of autophagosomes was counted in each view. A reduced number of autophagosomes were observed in the EP and EP-sham-DBS groups compared with the control group, which was increased by ANT-DBS. (n=3 in each group; in each monkey, twenty cells were randomly selected, and the average number of autophagosome for each monkey was recorded) *P < 0.05; **P < 0.01; ***P < 0.001; NS, P > 0.05. Data were presented as mean ± SD. I, control group; II, EP group; III, EP-sham-DBS group; IV, EP-DBS group.
The number of autophagosomes was also measured by TEM. Fewer autophagosomes were observed in the EP and EP-sham-DBS groups compared with the control group. Nevertheless, ANT-DBS induced a marked increase in the number of autophagosomes (F(3,8)= 8.636, P < 0.01) (Figure 6D and 6E).
We next measured the co-localization of LC3 and LAMP-1, a marker of lysosomes, in the hippocampus. Co-localization of the two proteins was decreased in hippocampal CA1 (F(3,8)= 26.604, P < 0.001) and CA3 (F(3,8)= 19.519, P < 0.001) neurons of the EP and EP-sham-DBS groups, but the situation was reversed in the EP-DBS group (Figure 7). Taken together, these results suggest that ANT-DBS stimulates autophagy by promoting autophagosome formation in epilepsy.
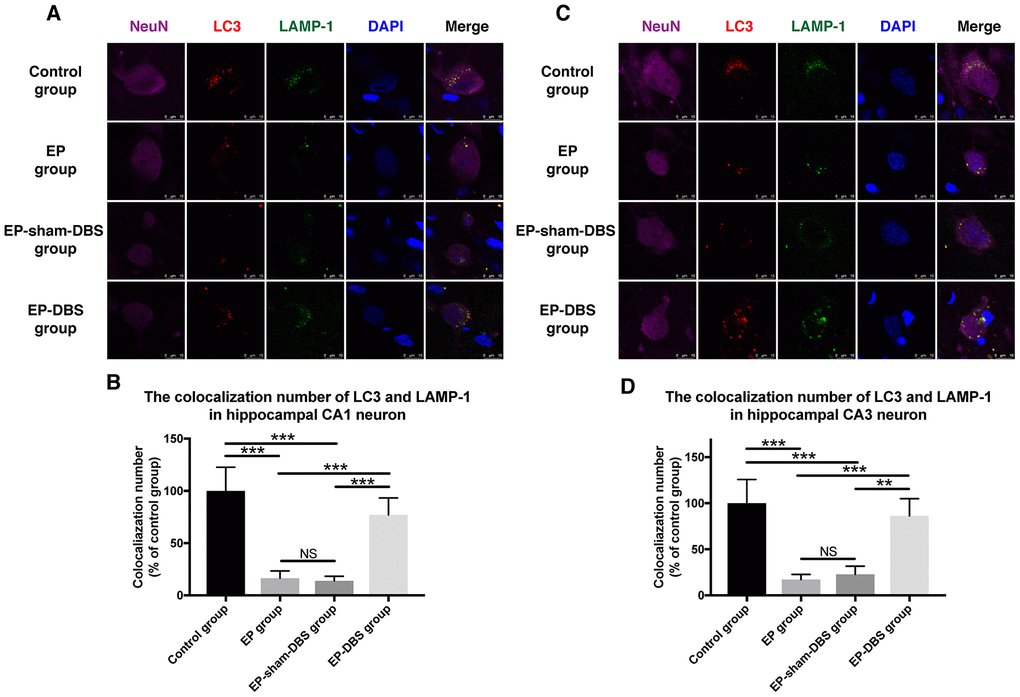
Figure 7. ANT-DBS increased the colocalization of LC3 and LAMP1 in the hippocampal neurons of epileptic monkeys. Colocalization of LC3 and LAMP-1 was decreased in hippocampal CA1 (A) and CA3 (C) neurons in EP and EP-sham-DBS groups, and increased by ANT-DBS. Colocalization number of LC3 and LAMP-1 in hippocampal CA1 (B) and CA3 (D) neurons. (n=3 in each group; in each monkey, a total of thirty cells in CA1 or CA3, the average immunofluorescent intensity of these cells was recorded) **P < 0.01; ***P < 0.001; NS, P > 0.05. Data were presented as mean ± SD.