Introduction
Ischemic stroke is the single most devastating neurological disease and a leading cause of death worldwide [1]. Clinical treatment by means of tissue plasminogen activator (tPA)-mediated thrombolysis and endovascular thrombectomy has significantly improved options within the acute time window of ischemic attack. In rodent middle cerebral artery occlusion (MCAO) models, the injury is characterized by the necrosis of neurons of the cerebral cortex caused by the combination of extensive inflammation and oxidative stress [2]. Moreover, cumulative evidence has shown that the consumption of antioxidants and overproduction of reactive oxygen species (ROS) are associated with the severity and progression of CIRI [3]. Although the neuroprotective effects of a great deal of antioxidant agents have been identified in rodent models, most have failed in subsequent clinical trials. Thus, explorations of the in-depth neuropathological mechanisms of ischemic stroke and the development of new therapeutic strategies for ischemic stroke present great challenges.
Coicis Semen, a polyphenolic compound extracted from Coix, is widely consumed as food and medicine in Asian countries. As a traditional Chinese medicine, Coicis Semen is thought to possess antioxidant, immunological, and anti-inflammatory effects and has been used to treat diabetic nephropathy [4], gastrointestinal diseases [5], skin diseases [6], and even tumors [7–9]. However, whether Coicis Semen exerts a neuroprotective effect against ischemic stroke needs to be investigated.
Angiogenesis is the physiological process by which new microvessels branching from pre-existing vessels are formed [10]. After ischemic stroke, the blood-brain barrier (BBB) is acutely disrupted, resulting in secondary brain injury due to an increase in the degree of cerebral edema, causing the infiltration of peripheral immune cells and exacerbating the inflammatory response in the central nervous system (CNS) [11]. Accumulating evidence indicates that angiogenesis not only alleviates BBB leakage [12] but also contributes to neuronal metabolism and remodeling [13]. In rodent MCAO models, endothelial cells (ECs) start to proliferate as early as 12-24 h after ischemia, but the reformation of cerebral microvessels in the penumbra and core of the ischemic infarct occurs within 4 to 7 days [14, 15]. Meanwhile, neuronal remodeling and axonal outgrowth do not occur until 14 days after stroke [15]. Thus, the promotion of angiogenesis may become a promising therapeutic strategy for the restorative treatment of ischemic stroke. Transforming growth factor-β (TGF-β) signaling pathways are involved in regulating EC proliferation and angiogenesis [16, 17]. Activin receptor-like kinase-1 (ALK1) is a surface receptor for the TGF-β1 protein that is preferentially expressed in ECs. During angiogenesis, ALK-1 is activated by binding TGF-β, which then induces the phosphorylation of Smad1/5/8, which dimerize with Smad4; this complex is then transferred to the nucleus, where it regulates the transcription of angiogenesis-related genes [18, 19]. Several studies found that the deletion of any components of the TGF-β1/ALK1/Smad1/5/8 pathway resulted in abnormalities in primitive vascular plexus formation and embryonic lethality in mice due to defects in angiogenesis [20, 21]. In addition, studies have demonstrated that the TGF-β/ALK5 signaling pathway plays a critical role in neurogenesis and functional recovery following ischemic stroke [22, 23]. Thus, these observations led us to wonder whether Coicis Semen can regulate angiogenesis via the TGF-β1/ALK1/Smad1/5/8 signaling pathway following stroke, which remains unknown.
In this study, we generated a mouse MCAO model and oxygen-glucose deprivation/reoxygenation (OGD/RX) model in human umbilical vein endothelial cells (HUVECs) to investigate whether Coicis Semen has a neuroprotective effect and its potential molecular effects on ischemia-induced oxidative stress and angiogenesis. Our results demonstrate that Coicis Semen may protect against cerebral ischemic injury in the brain by inhibiting oxidative stress and promoting angiogenesis via activation of the TGFβ1/ALK-1/Smad1/5/8 signaling pathway.
Results
Coicis semen reduced infarct volume, ameliorated neurological deficits and reduced edema after 3 days of MCAO
To examine whether Coicis Semen has a neuroprotective effect and determine its optimal dose in MCAO mice, we established a mouse MCAO model and measured infarct volume, neurological deficit outcomes and the water content of the brain. Neurological scoring was performed 3 days after MCAO. TTC staining of all brain slices showed that the administration of Coicis Semen (50, 100, and 150 mg/kg) significantly reduced infarct volume compared with that of the MCAO + vehicle group (P < 0.05) (Figure 1A, 1B). Similarly, neurological scores and the cerebral water content were significantly reduced in the Coicis Semen-treated groups compared with the vehicle-treated group (P < 0.05) (Figure 1C, 1D). Additionally, Coicis Semen at a dose of 100 mg/kg dose had the greatest neuroprotective effect, and this dose was selected for subsequent experiments.
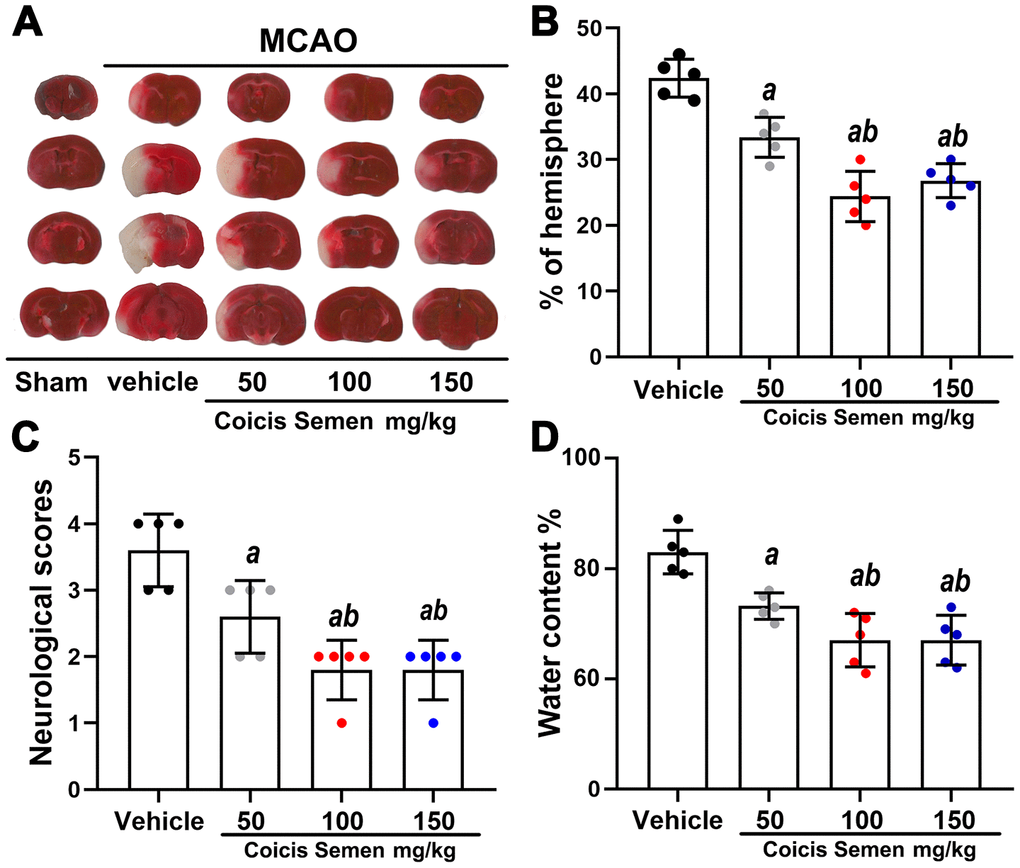
Figure 1. Coicis semen treatment reduced the infarct volume, improved neurological function and decreased the brain water content in MCAO mice. (A) TTC staining of brain sections from vehicle- or Coicis Semen-treated mice taken at 3 days poststroke. (B, C, D) Effects of Coicis Semen at different concentration on infarct volume, neurological score and brain water content in mice at 3 days poststroke. Mean ± SD. n = 5. aP < 0.05 vs. vehicle; bP < 0.05 vs. Coicis Semen (50 mg/kg). Statistical analyses were performed by one-way ANOVA followed by Tukey’s multiple comparison tests.
Coicis semen promoted long-term neurological recovery after ischemic stroke
Although ischemic injury induces neurological function deficits in the acute stage of stroke, recovery after the injury is possible. Therefore, we explored the long-term neuroprotective effect of Coicis Semen on stroke. We detected the relative infarct volume, survival rate, neurological scores and behavioral functions of the MCAO mice on days 3, 7 and 14 after MCAO. Compared with vehicle administration, Coicis Semen administration significantly reduced the relative infarct volume at each time point (P< 0.05, Figure 2A, 2B). Moreover, the neurological scores were also significantly decreased on days 3, 7, and 14 after MCAO mice were treated with Coicis Semen (P< 0.05, Figure 2C). With prolonged reperfusion, the neurological function of mice in the MCAO + vehicle group gradually recovered. Additionally, the survival rates of the Coicis Semen groups were remarkably elevated compared with that in the MCAO + vehicle group (Figure 2D). Furthermore, Coicis Semen significantly reduced motor deficit, as shown by the increase in the latency to fall from the rotarod and pole at each time point after stroke compared with that in the MCAO + vehicle group (P< 0.05, Figure 2E, 2F).
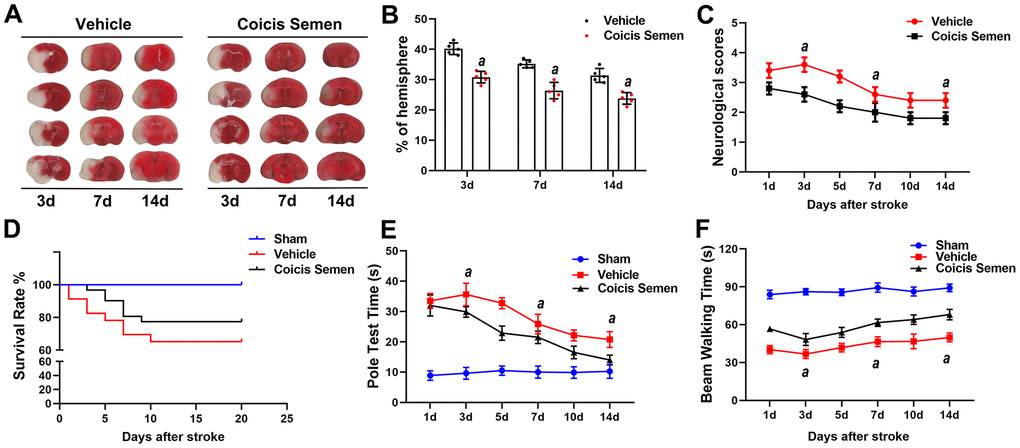
Figure 2. Coicis semen treatment promoted long-term neurological recovery after ischemic stroke. (A) The ipsilateral infarct volume was detected at 3, 7 and 14 days after stroke by using TTC staining and then normalized to the infarct volume of the contralateral hemisphere to correct for edema; the data are expressed as a percentage. (B) Bar graphs show quantified infarct volumes. n =5, aP < 0.05 vs. vehicle. Statistical analyses were performed by two-tailed Student’s t-test. (C) Neurological deficit scores. n =5. (D) Survival rate. n = 15-20 (E) Pole test time. (F) Beam walking time. Mean ± SD. n = 10-12. aP < 0.05 vs. vehicle by one-way ANOVA followed by Tukey’s multiple comparison tests (individual time points).
Coicis semen decreased oxidative stress following ischemic stroke
To understand the neuroprotective mechanism of Coicis Semen, we focused on its antioxidation effect. After stroke, oxidative stress is activated, and a large amount of ROS is produced. SOD, GSH, and MDA are vital biomarkers of oxidative stress, and their levels indirectly reflect the degree of lipid peroxidation and directly show the level of free radical scavengers. We found that the production of ROS in the brain was markedly increased at 3 days after stroke and had gradually decreased on the 7th and 14th days following stroke. Furthermore, Coicis Semen treatment significantly decreased the number of ROS-positive cells compared to those in the MCAO + vehicle group (P< 0.05, Figure 3A, 3B). The Coicis Semen-treated groups showed reduced levels of MDA in comparison to the MCAO + vehicle group (P< 0.05, Figure 3C). Meanwhile, SOD and GSH levels were remarkably lower in the MCAO + vehicle group than in the Coicis Semen-treated groups (P< 0.05, Figure 3D, 3E). Taken together, these data suggest that Coicis Semen can inhibit oxidative stress activation in ischemic stroke.
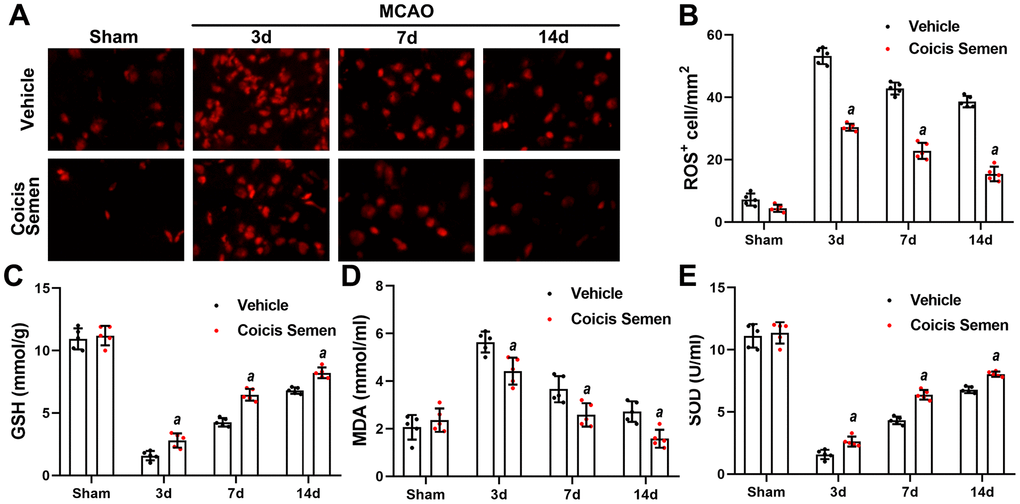
Figure 3. Coicis semen treatment reduced oxidative stress levels after ischemic stroke. (A) Changes in the production of ROS were revealed by DHE staining. Scale bar =50 μm. (B) Quantification of ROS-positive cells in the brain. n = 5, aP < 0.05 vs. vehicle. Statistical analyses were performed by two-tailed Student’s t-test. (C, D, E) The levels of GSH, MDA and SOD were quantified by using commercial kits. Mean ± SD. n = 5. aP < 0.05 vs. vehicle. Statistical analyses were performed by two-tailed Student’s t-test.
Coicis semen attenuated ischemic stroke-induced BBB damage
Since the BBB plays a crucial role in the long-term effects of ischemic stroke, we investigated the effect of Coicis Semen on the recovery of BBB function. On days 7 and 14 after MCAO, we evaluated the BBB permeability of mice by EB dye staining. Under normal conditions, EB dye cannot enter the brain due to the protection conferred by the BBB. More whole-brain vascular leakage was found in mice in the MCO + vehicle group than in mice in the Coicis Semen-treated groups (Figure 4A). Moreover, EB extravasation was reduced in the Coicis Semen-treated groups at each time point after stroke compared with that in the MCAO + vehicle group (P< 0.05, Figure 4B). The BBB is composed of ECs, astrocytic end-feet, pericytes, and tight junction proteins, including ZO-1 and Occludin. Thus, we used western blot analysis to detect the expression of ZO-1 and Occludin, the results of which showed that the levels of both ZO-1 and occludin were significantly increased in Coicis Semen-treated MCAO mice compared with vehicle-treated MCAO mice (P< 0.05, Figure 4C–4E).
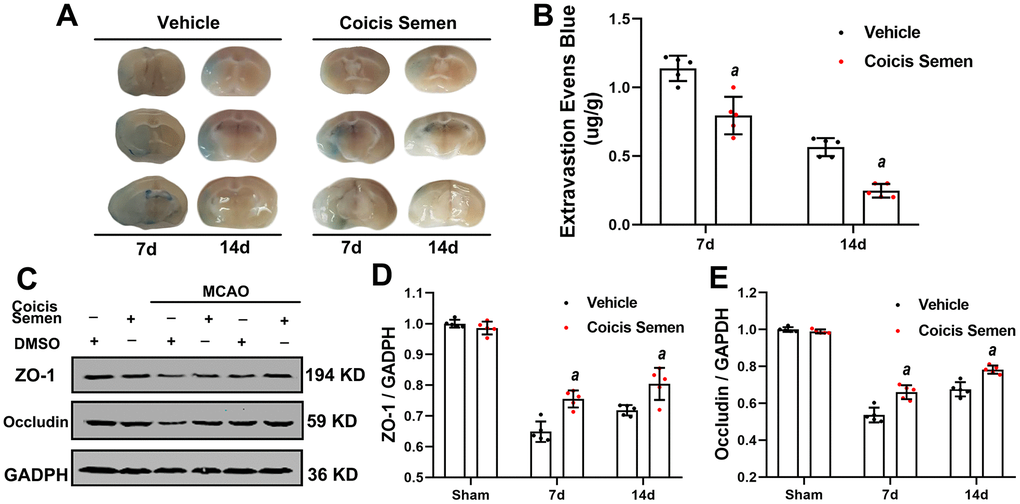
Figure 4. Coicis semen alleviated blood-brain barrier (BBB) leakage after ischemic stroke. (A) Images showing Evans blue (EB) dye leakage in brains after EB injection (by tail vein). (B) EB dye extravasation 2 h after tail vein injection of EB dye was quantitatively analyzed using a spectrophotometer. (C) Western blot analysis showing that Coicis Semen treatment increased the expression of ZO-1 and Occludin. (D) Quantitative analysis of the ZO-1 protein levels. (E) Quantitative analysis of the Occludin protein levels. Mean ± SD. n = 5. aP < 0.05 vs. vehicle. Statistical analyses were performed by two-tailed Student’s t-test.
Coicis semen promoted angiogenesis in the ischemic penumbra of MCAO mice
The improvement in stroke outcomes in Coicis Semen-treated mice led to us to hypothesize that Coicis Semen improves angiogenesis and cerebral blood flow during stroke recovery. To test this hypothesis, we measured expression of the angiogenesis-related proteins CD31 and VEGF using immunofluorescence and ELISA. The immunofluorescence results showed that Coicis Semen treatment significantly increased the proportion of CD31-positive cells in the penumbra of the ischemic brain compared to that in the MCAO + vehicle group after 3, 7, or 14 days of reperfusion after MCAO (P< 0.05, Figure 5A, 5B). Furthermore, Coicis Semen administration also considerably improved VEGF levels in the penumbra of the ischemic brains of MCAO mice at 3, 7 and 14 days after reperfusion following MCAO compared to those in the ischemic brains of vehicle-treated mice (P< 0.05, Figure 5C).
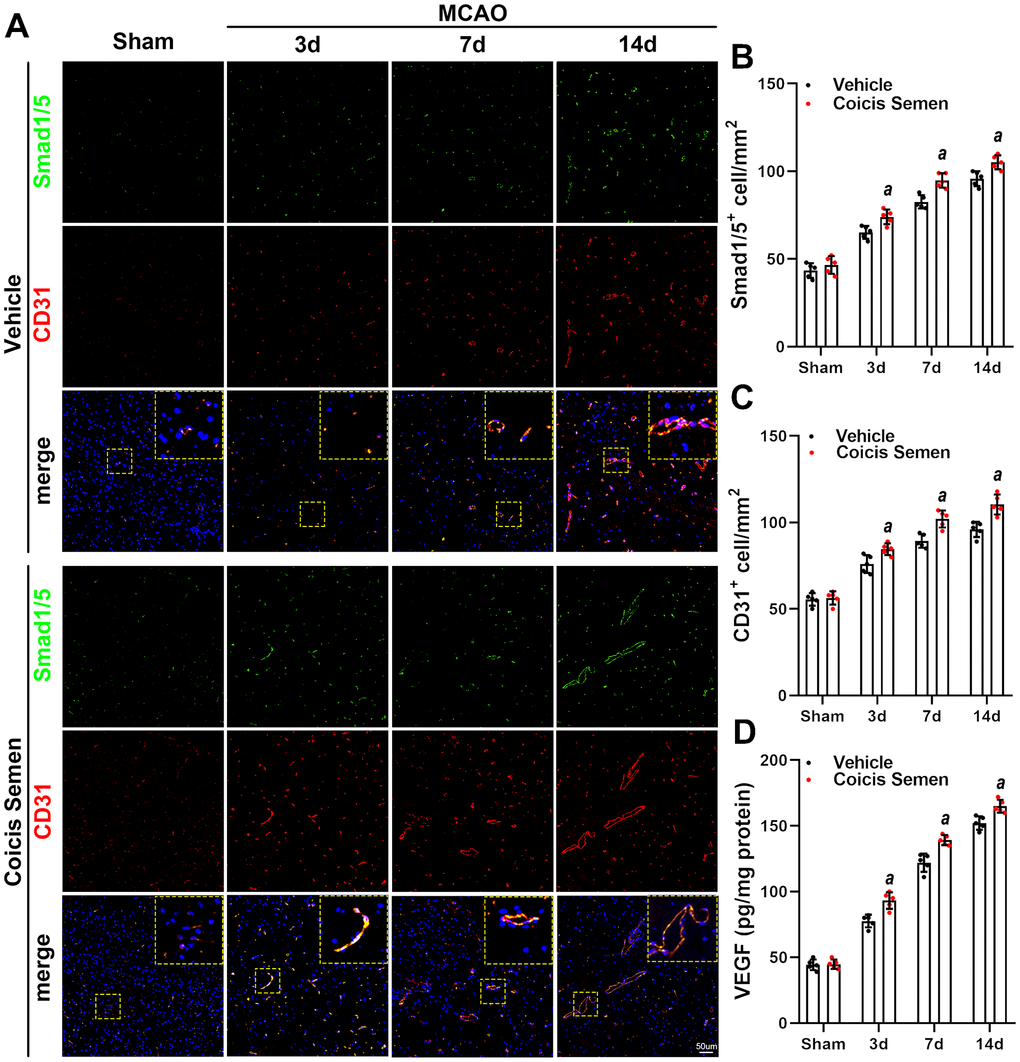
Figure 5. Coicis semen promoted angiogenesis after ischemic stroke. (A) Images showing CD31 detection in the ischemic penumbra via immunofluorescent staining and counterstaining with DAPI. (B) Quantification of CD31-positive cells in the ischemic penumbra. (C) VEGF levels were detected by ELISA. Mean ± SD. n = 5. aP < 0.05 vs. vehicle. Statistical analyses were performed by two-tailed Student’s t-test.
Coicis semen potentiated TGF-β1/ALK1/Smad1/5 signaling pathway activation after stroke
To further explore the molecular signaling mechanism by which Coicis Semen attenuated ischemic-induced BBB injury and promoted angiogenesis, we measured the expression of members of the TGF-β1/ALK1/Smad1/5 signaling pathway, a key intracellular signaling system that plays a pivotal role in the proliferation of the endothelium and regulation of angiogenesis in brain recovery following ischemic stroke. CD31 is mainly expressed in the endothelium and plays a key role in the generation of new blood vessels. Thus, immunofluorescence showed that the number of Smad 1/5-positive cells colocalized with ECs (CD31-positive cells) in the infarct zone was significantly increased in mice treated with Coicis Semen compared to mice treated with vehicle (P< 0.05, Figure 5A, 5C). Additionally, western blot analysis of cerebral protein extracts was applied to assess the levels of CD31 and TGF-β1 signaling-associated proteins. CD31, TGF-β1, ALK-1, Smad1/5 and p-Smad1/5 levels in the penumbra of the ischemic brain were elevated at 7 and 14 days after stroke, and this increase was significantly improved by Coicis Semen administration (P < 0.05, Figure 6). Overall, our results indicated that Coicis Semen treatment could effectively improve angiogenesis and activate the TGF-β1 pathway in MCAO mice during the recovery phase.
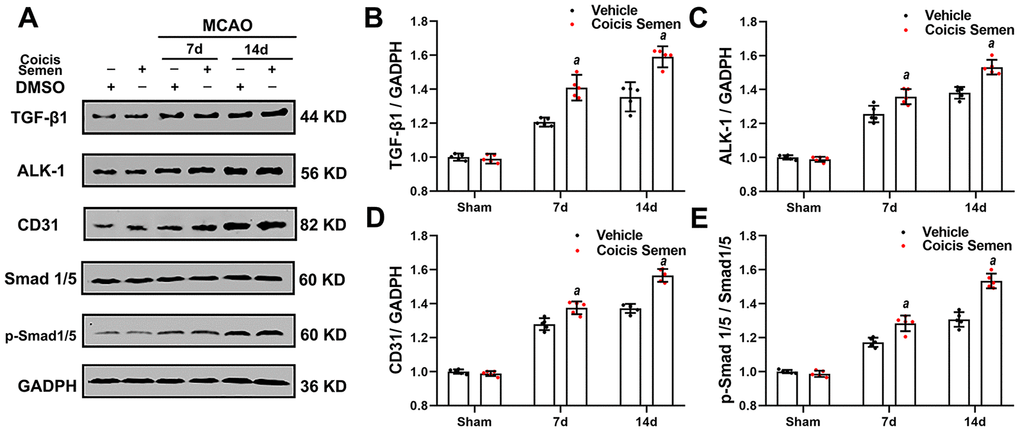
Figure 6. Coicis semen treatment activated the TGF-β1-ALK1-Smad1/5 signaling pathway after stroke. (A) Western blot showing that Coicis Semen treatment increased the expression and phosphorylation of the TGF-β1-ALK1-Smad1/5-associated proteins TGF-β1, ALK-1, Smad 1/5, p-Smad 1/5 and CD31 at 7 and 14 days after stroke. (B–E) Quantitative analysis of the protein levels of TGF-β1, ALK-1, p-Smad 1/5 and CD31 in ischemic cortical tissue. Mean ± SD. n = 5. aP < 0.05 vs. vehicle. Statistical analyses were performed by two-tailed Student’s t-test.
Coicis semen increased cell viability after OGD/RX and upregulated angiogenesis marker protein expression in HUVECs
ECs play a pivotal role in vascular remodeling and angiogenesis, but the role of Coicis Semen and its molecular signaling mechanism in ECs remain unknown. To address these issues, we used HUVECs to generate a stroke model by OGD/RX simulation in vivo. First, we performed a CCK-8 cytotoxicity assay to determine the optimal concentration of Coicis Semen to be administered to the HUVECs after OGD/RX. Coicis Semen at 400 μM, 500 μM, 600 μM and 700 μM effectively improved the relative viability of cocultured cells by comparing with DMSO group (P < 0.01, P < 0.01, P < 0.05, P < 0.05, Figure 7C), and we chose 500 μM (by compared with 400 μM, P < 0.01; and by compared with 600 μM, P < 0.05) as the optimal Coicis Semen concentration for subsequent experiments based on a previous study [24]. Then, we used immunofluorescence analysis to detect CD31 in HUVECs to confirm that Coicis Semen promoted angiogenesis in HUVECs after OGD/RX. The number of CD31-positive cells after OGD/RX was reduced in the vehicle-treated group compared to the sham group. Meanwhile, consistent with the in vivo results, Coicis Semen also increased the number of CD31-positive cells in the in vitro ischemia-reperfusion model (P < 0.05, Figure 7A, 7B).
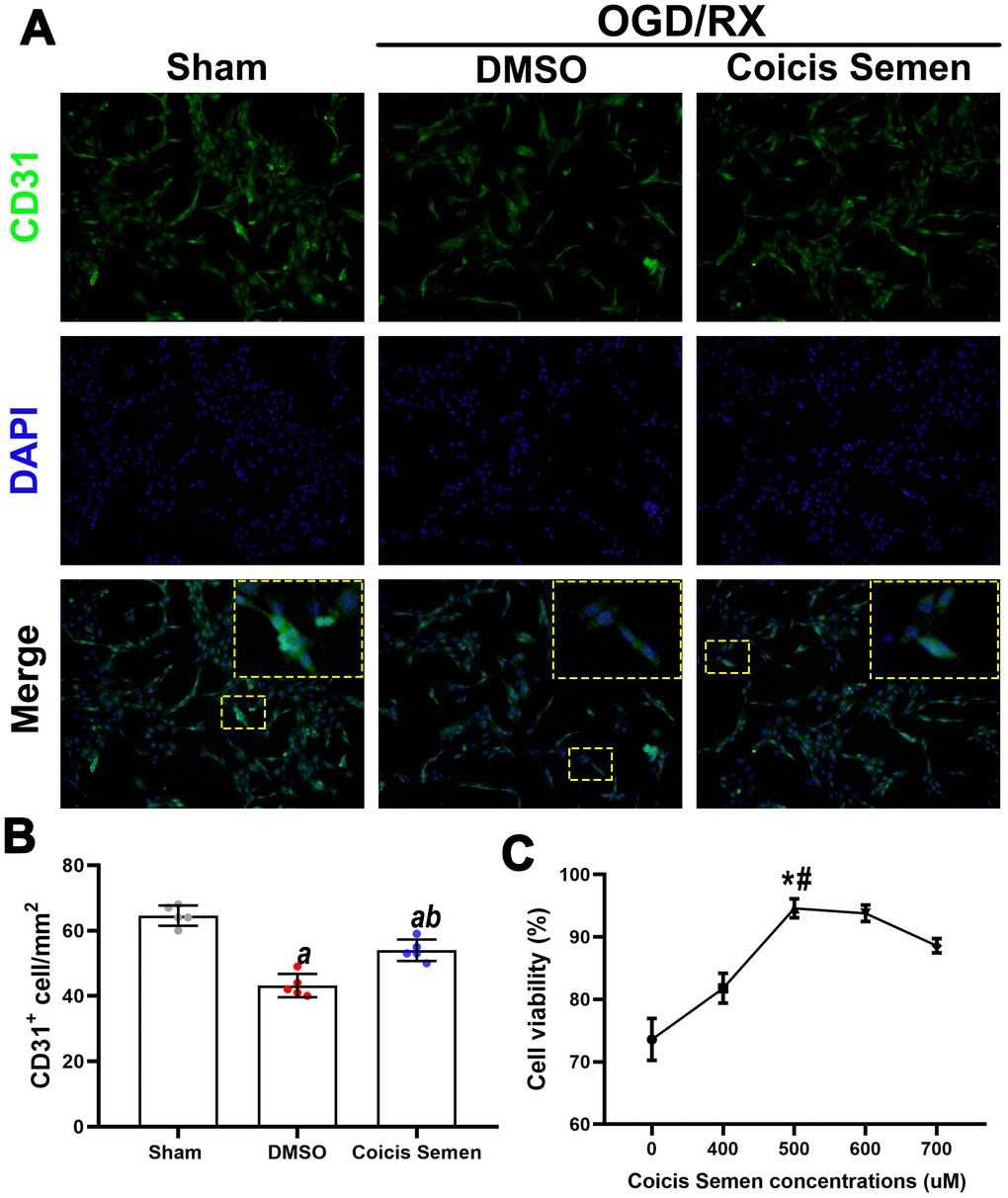
Figure 7. Coicis semen increased cell viability in HUVECs after OGD/RX. (A) Representative imaging showing CD31 detection in HUVECs after OGD/RX by using immunofluorescence staining. Mean ± SD. n = 5. aP < 0.05 vs. sham; bP < 0.05 vs. DMSO. Statistical analyses were performed by one-way ANOVA followed by Tukey’s multiple comparison tests. (B) Quantitative analysis of CD31-positive cells. (C) Quantitative analysis of cell viability in cells treated with Coicis Semen at different doses. Mean ± SD. n = 5. aP < 0.05 vs. sham; bP < 0.05 vs. DMSO; *P < 0.05 vs. Coicis Semen (400 μM); #P < 0.05 vs. Coicis Semen (600 μM). Statistical analyses were performed by one-way ANOVA followed by Tukey’s multiple comparison tests.
Coicis semen promoted angiogenesis via upregulation of the TGF-β1/ALK1/Smad1/5 pathway after OGD/RX in vitro
Based on the above results, we speculated that the neuroprotective effect of Coicis Semen is related to its antioxidative function and ability to promote angiogenesis via activation of the TGF-β1 signaling pathway. To confirm the molecular mechanism by which Coicis Semen promotes angiogenesis, we utilized LY2109761, an inhibitor of the TGF-β receptor kinase that can effectively block stimulation of p-Smad1/5 expression. Compared to that in the vehicle-treated group, the expression of TGF-β1 and its downstream target p-Smad1/5 in HUVECs after OGD/RX was downregulated after LY2109761 treatment (P < 0.01). However, their expression levels were upregulated by cotreatment with LY2109761 and Coicis Semen compared with those following treatment with LY2109761 alone. Then, we further measured the levels of VEGF, which is regulated by TGF-β1/ALK1/Smad1/5, and found that they were downregulated after inhibition with LY2109761 but increased by cotreatment with LY2109761 and Coicis Semen (P < 0.05) (Figure 8). These results strongly suggest that the protective effect of Coicis Semen on cerebral ischemia-reperfusion injury (CIRI) occurs through facilitating angiogenesis and inhibiting oxidative stress activation via upregulation of the TGF-β1/ALK1/Smad1/5 pathway.
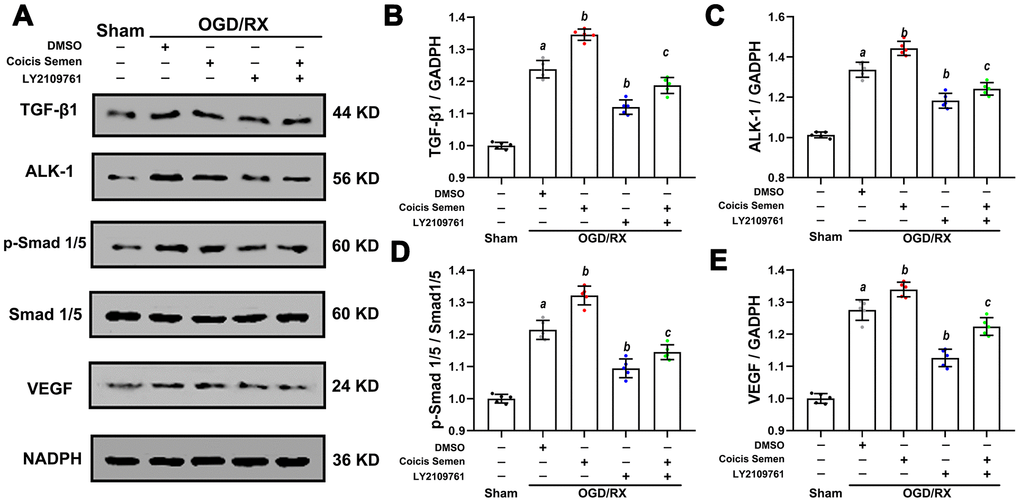
Figure 8. Coicis semen promoted angiogenesis in HUVECs after OGD/RX through upregulation of the TGF-β1-ALK1-Smad1/5 signaling pathway. (A) Representative western blots showing TGF-β1, ALK1, Smad1/5, p-Smad1/5 and VEGF. Quantitative analysis of TGF-β1 (B), ALK1 (C), p-Smad1/5 (D), and VEGF (E) protein expression levels in HUVECs after OGD/RX. Mean ± SD. n = 8. aP < 0.05 vs. sham; bP < 0.05 vs. DMSO + OGD/RX; cP < 0.05 vs. LY2109761 + OGD/RX. Statistical analyses were performed by one-way ANOVA followed by Tukey’s multiple comparison tests.
Discussion
Stroke is a major cause of disability and death worldwide, but the agents currently used to treat stroke are not sufficient to effectively improve the recovery of stroke patients [25, 26]. Coicis Semen, a traditional Chinese medicine, exerts antioxidant and anti-inflammatory effects in many diseases, including coronary heart disease [27], systemic inflammatory disease [28], and cancer [9, 29]. In a previous study, Coicis Semen was shown to have an antioxidant effect, and oxidative stress is closely associated with the prognosis of ischemic stroke [28, 30]. This led us to speculate that Coicis Semen also protects against CIRI. Here, for the first time, we show that Coicis Semen has a neuroprotective effect against ischemic stroke by inhibiting oxidative stress activation and promoting angiogenesis. First, we successfully established CIRI models in vivo and in vitro, after which we measured the infarct volume, cerebral edema, neurological function, survival rate and behavior from 3 to 14 days following stroke in vivo and cell viability in vitro. Coicis Semen significantly increased the survival rate of MCAO mice, reduced the infarct volume, alleviated edema, improved neurological function and increased the cell viability of HUVECs after OGD/RX. Second, to explore the neuroprotective mechanisms of Coicis Semen, we detected the levels of oxidation and antioxidant indicators, ROS, SOD, GSH and MDA, in the brain and assessed BBB function as well as angiogenesis levels. Coicis Semen dramatically inhibited oxidative stress activation, alleviated BBB leakage and promoted angiogenesis in CIRI. Third, to further explore the molecular signaling mechanism by which Coicis Semen promotes angiogenesis and inhibits oxidative stress activation, we measured the expression of TGF-β1 and its downstream proteins, which have well-established effects on angiogenesis. Coicis Semen treatment significantly activated the TGF-β1/ALK1/Smad1/5 pathway in the recovery phase of stroke. Furthermore, an in vitro study confirmed that Coicis Semen treatment significantly increased EC viability and promoted angiogenesis via the activation of TGF-β1/ALK1/Smad1/5 signaling after ischemic injury, facilitating facility after CIRI.
In the acute phase of ischemic stroke, cerebral infarction, neurological function deficit and BBB damage are triggered by mitochondrial dysfunction, the inflammatory cascade and energy metabolism disorders, resulting in the overproduction of oxygen free radicals [31]. Accumulating evidence has confirmed that excess ROS may inhibit antioxidant enzyme activity, mediate oxidative damage to DNA/RNA, and finally induce neuronal apoptosis [32]. Although ROS at moderate concentrations are important for physiological processes, excessive ROS generation and antioxidant consumption contribute to secondary brain injury [33]. As the brain is the part of the body most susceptible to oxidative stress, several studies have found that an increase in oxygen free radical formation leads to a larger infarct volume and more severe neurologic deficit [34–36]. Moreover, antioxidant (SOD and GSH) consumption as well as malondialdehyde (MDA) accumulation directly reflect the degree of lipid peroxidation in ischemic stroke. Polysaccharides and polyphenols, major active components of Coicis Semen, are thought to have immunological, antioxidant, and anti-inflammatory effects [27, 37, 38]. Importantly, in previous studies, Coicis Semen was shown to decrease the levels of GSH and SOD and increase serum MDA levels in rats with rheumatoid arthritis [28]. Thus, in the present study, we examined the levels of ROS, SOD, GSH and MDA in the brains of MCAO mice to measure the degree of oxidative stress. We found that ischemic stroke initiated oxidative stress activation, generated excess ROS and MDA, and decreased the levels of SOD and GSH, but all of these effects were inhibited by Coicis Semen treatment. Thus, the neuroprotective effect of Coicis Semen may be related to the inhibition of oxidative stress activation during the acute phase of ischemic stroke.
Angiogenesis is a vital protective and restorative mechanism in response to ischemic stroke [39]. Occurring in the penumbra region as early as 12 h after stroke, angiogenesis was shown to last for more than 21 days following cerebral ischemia in rodent experiments [40, 41]. Angiogenesis in the ischemic boundary region after ischemic stroke contributes to the reformation of neuropil networks and restoration of blood flow and neuronal metabolism in tissue at risk [42]. Consistent with previous studies [13], our findings also confirmed that the expression of CD31 (an angiogenesis marker) gradually increased throughout a prolonged recovery period (1-14 days after MCAO). Furthermore, Coicis Semen treatment accelerated microvessel remodeling during the recovery phase of ischemic stroke (7 and 14 days after MCAO). In the recovery phase of stroke, angiogenesis is triggered by the proliferation and differentiation of vascular smooth muscle cells and the activation of matrix metalloproteinases. Additionally, the BBB, a protective barrier of the CNS, is constructed by ECs, astrocytic end-feet, pericytes, and tight junction proteins. Ischemia-induced BBB breakdown leads to cerebral edema, hemorrhage and an inflammatory cascade, resulting in secondary brain injury [43]. In this study, we found that BBB leakage gradually improved with prolonged recovery time after stroke, and Coicis Semen improved the recovery of BBB function. New blood vessel generation contributed to BBB functional recovery and improved the prognosis of stroke. Consistent with the neurological function improvement observed at 7 and 14 days in the MCAO mice, Coicis Semen not only attenuated ischemia-induced BBB leakage but also promoted angiogenesis in the penumbra of the ischemic region at 7 and 14 days after stroke.
Many molecules, including insulin-like growth factor (IGF-1), epidermal growth factor (EGF), erythropoietin (EPO) and hepatocyte growth factor (HGF), are known to regulate angiogenesis. A signaling pathway involving VEGF was identified as a key player in the regulation of angiogenesis [44]. In our study, we found that Coicis Semen could increase the levels of VEGF at 7 and 14 days after stroke and elevate the viability of ECs after OGD/RX. Many signaling pathways are involved in the regulation of VEGF. In a previous study, the TGF-β1-ALK1-Smad1/5 signaling pathway was confirmed to play an important role in angiogenesis and the regulation of VEGF in ECs and mural cells [45]. The TGF-β superfamily consists of multifunctional cytokines that exerts their effects by binding specific type I and type II serine or threonine kinase receptors and transferring intracellular Smad transcription factors to the nucleus. ALK-1 is a type І receptor of TGF-β that is mainly expressed on ECs [46]. Upon ALK-1 activation, receptor-regulated Smad1 and 5 are recruited to ALK-1 and phosphorylated by ALK-1 at two serine residues, after which they are translocated to nucleus and then stimulate EC migration, proliferation and tube formation [47]. Additionally, TGF-β1 has been reported to protect hippocampal neurons from multiple types of damage, including oxidative stress and excitotoxic and ischemia injury [48]. A recent study in models of brain ischemia demonstrated that the TGF-β1 signaling pathway is involved in brain ischemia by regulating neurogenesis and angiogenesis [49]. Our findings revealed that ischemic stroke initiated TGF-β1-ALK1-Smad1/5 pathway activation and increased the levels of VEGF, but these changes were improved by Coicis Semen treatment. To further support the effect of Coicis Semen in activating the TGF-β1-ALK1-Smad1/5 signaling pathway, LY2109761 was utilized. In in vitro OGD/RX experiments, we found that Coicis Semen also activated the TGF-β1-ALK1-Smad1/5 signaling pathway after its inhibition by LY2109761 in ECs. These findings suggest that Coicis Semen exerts a neuroprotective effect in the ischemic brain partly by activating the TGF-β1-ALK1-Smad1/5 pathway, subsequently improving angiogenesis and BBB function.
The central finding of our study is that Coicis Semen reduces brain infarction by inhibiting oxidative stress activation, restoring the BBB and promoting angiogenesis; these effects are related to TGF-β1-ALK1-Smad1/5 signaling pathway activation. Over recent years, most neuroprotective agents assessed in previous studies have been designed to target pathological cascades in ischemic stroke (such as anti-inflammatory glutamate antagonists), apoptosis and necrosis [50, 51]. However, most neuroprotective agents have some side effects, and clinical therapy with these agents is limited. Our current study shows that Coicis Semen, a common food in Asian countries, can protect against CIRI and may provide a rationale in support of its clinical translation.
Materials and Methods
Animals
Wild-type C57BL/6J mice (n = 180, when animals that died and animals in which modeling was unsuccessful due to the absence of infarction or infarction with hemorrhage were excluded (n=77), 25-30 g) were obtained from Hubei Experimental Animal Research Center (Hubei, China; Nos. 43103740114827, 43103740014717, 43203440117027). The animals were house in an environment with controlled humidity (60 ± 5%) and temperature (21 ± 2° C) under a 12/12-h light/dark cycle and given free access to standard laboratory food and water for at least 1 week before the experiments. All animal experimental protocols were approved by the Animal Experimentation Ethics Committee of Wuhan University and conducted according to the Animal Care and Use Committee guidelines of Renmin Hospital of Wuhan University.
Drug administration and experimental grouping
Coicis Semen (100 g; S27205, Yuanye Bio-Technology, Shanghai, China) was prepared in 100 mM dimethyl sulfoxide (DMSO) to the desired concentrations. Before MCAO, mice received Coicis Semen at different concentrations by oral administration for 28 days as previously described [27, 28]. The mice were randomly divided into the following groups: the sham, MCAO + vehicle, MCAO + Coicis Semen (50 mg/kg), MCAO + Coicis Semen (100 mg/kg), and MCAO + Coicis Semen (150 mg/kg) groups. After the appropriate doses of Coicis Semen were chosen at 3 days after MCAO, we administered Coicis Semen at these concentrations to MCAO mice for 7 and 14 days. A vehicle solution containing no Coicis Semen was administered to the MCAO + vehicle group.
MCAO modeling and infarct volume measurement
The mice were subjected to MCAO, the method that produces injury most similar to human stroke, as previously described [12, 52]. Briefly, wild-type C57BL/6J mice were anesthetized with 5% isoflurane in O2 with a facemask, and the left middle cerebral artery was ligated with 6-0 monofilament nylon (Doccol, Corp., Redlands, CA, United States). After 1 h of occlusion, the monofilament nylon was removed, and reperfusion was initiated. The mice were placed on a homeothermic heating pad (TMS-202, Softron Biotechnology, Beijing China) to stabilize their body temperature at 37 ± 0.5° C. Mice in the sham group underwent the same procedure without monofilament ligation. The mice were deeply anesthetized and euthanized with an overdose of isoflurane on days 3, 7 and 14 after MCAO. The brain tissues were cut into 2-mm coronal sections and then stained in 2% 2,3,5-triphenyltetrazolium chloride (TTC) (17779, Sigma-Aldrich, United States). The infarct volume was detected and analyzed by a blinded observer using ImageJ v1.37 (NIH, Bethesda, MA, United States) as described previously [53] and normalized and is presented as a percentage of the volume in the nonischemic hemisphere to correct for edema.
Assessment of neurological deficit
Neurological deficit scores were evaluated from 1 to 14 days after MCAO as described previously [54, 55]. The scores ranged from 0 (no observable neurological deficits) to 4 (no spontaneous motor activity and loss of consciousness) and were measured with a method from Longa EZ [56].
Brain water content
Cerebral edema was assessed 3 days after reperfusion by the wet/dry method as previously described [57]. In brief, the brains were removed without perfusion, and the wet weights were measured on an electronic balance. Dry weights were measured after heating the specimens at 105° C for 24 h. The brain water content was calculated by using the following formula: (wet weight-dry weight)/wet weight × 100%.
Rotarod test
The time that each mouse spent on a rotarod was measured with an accelerating rotarod (SD Instruments, San Diego, CA). Before MCAO, each mouse was trained for 9 days, and the rotation speed was increased from 4 to 8 rpm every 4 min. The time between the placement of the mouse on the pole and its falling off the rod was recorded. After the training period, the mice in each group performed the test on days 1, 3, 5, 7, and 14 after stroke. The times were calculated by averaging the times recorded for each mouse in three experiments.
Beam-walking tests
Beam-walking tests were performed using wooden beams of cylindrical ridged beam 8 mm in diameter and the beam was 30 cm long and placed 30 cm above the bench top. The times were calculated according to the ability of the mouse to walk along the beam from one end to the other.
Pole test
Each mouse was placed on a 50-cm vertical pole with its head upward. The time that the mouse spent on the pole before falling to the cage floor was recorded. Before MCAO, each mouse was trained for 3 days (5 trials per day). After the training period, the mice in each group were tested on days 1, 3, 5, 7, and 14 after stroke. The times were calculated by averaging the three times recorded for each mouse in three experiments.
Measurement of ROS, SOD, GSH and MDA levels
To assess ROS production, the brain was carefully and quickly isolated, cut into 4.0-μm sections with a freezing microtome (CryoStar NX50, Thermo, USA, MA) and placed on chilled microscope slides. The samples were incubated in physiological saline solution (PSS) containing 10 μmol of dihydroethidium (DHE; Sigma-Aldrich) for 30 min at 37° C in a dark room. The brain was washed twice with PSS and placed under an automatic fluorescence microscope (BX63, Olympus Optical Ltd., Tokyo, Japan). The brain tissue was removed and immediately dissected on a cooled ice pack (−20° C) at the indicated time after reperfusion. Brain SOD, GSH and MDA levels were measured by SOD, GSH and MDA assay kits, respectively, following the manufacturer's directions (A001-3-2, A006-2-1, A005-2-1, Nanjing Jian Cheng Bioengineering Institute, Nanjing, China).
BBB permeability assay
Evans blue (EB; 2% in PBS; Sigma-Aldrich, E2129) dye was injected via the tail vein at 4 h before the mouse was sacrificed. The entire brain tissue was homogenized and incubated in formamide (24 h, 55° C), and the absorbance of the homogenate supernatant at 620 nm was measured. The EB concentrations were normalized based on the results from brain samples from the sham-operated mice. The results were calculated using a standard curve of EB in formamide and are presented in units of micrograms per gram of brain tissue.
VEGF ELISA
A homogenate of the infarct area of the brain tissue containing VEGF was centrifuged (1200 ×g, 10 min, 4° C), and the concentrations of VEGF in the supernatant was measured with an ELISA kit according to the manufacturer’s instructions (MMV00, R&D Systems, Minnesota, USA).
Immunofluorescence staining
The mice were euthanized, perfused with cold PBS, and fixed with 4% paraformaldehyde for 2 days. Paraffin sections were then prepared from the brains. Before staining, the sections were deparaffinized and rehydrated, and antigen retrieval was carried out, followed by treatment with 0.3% hydrogen peroxide to quench endogenous peroxidase activity. The cells were adhered to plates before staining and washed 3 times with cold PBS. Afterward, the slices and cells were blocked with 0.1 M PBS containing 5% fetal bovine serum (FBS) and 0.3% Triton X for 1 h at room temperature. After washing, the slices were incubated at 4° C overnight with the following primary antibodies: anti-CD31 (1:200, ab28364, Abcam, Cambridge, United Kingdom) and anti-Smad 1/5 (1:200, abs130460, Absin, Beijing, China). The slices were then rinsed and incubated with Alexa594-conjugated anti-CD31 antibody (1:200, ANT030, Millipore, Billerica, MA, United States) or Alexa 488-conjugated anti-Smad1/5 antibody (1:200, ANT024, Millipore, Billerica, MA, United States) for 2 h at room temperature. After thorough rinsing, the nuclei were stained with DAPI (94010, Vector Laboratories, Burlingame, CA, United States). All slices were photographed using an automatic fluorescence microscope (BX63, Olympus Optical, Ltd., Tokyo, Japan). The numbers of immunoreactive cells in predefined areas were quantified using ImageJ software (Media Cybernetics, Inc., Rockville, MD, United States). Cells in six different fields per mouse in six mice per group were counted. All counts were conducted by blinded observers.
In vitro cell culture and OGD/RX
HUVECs were obtained from the China Center for Type Culture Collection (Wuhan, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS (#13011-8611, Zhejiang Tian Hang Biotechnology, Co., Ltd., Zhejiang, China) and 1% antibiotic (GNM15140, Genome, China) in an incubator supplied with 5% CO2 at 37° C. Before OGD/RX injury was induced, the cultured cells in the logarithmic growth phase were rinsed twice with PBS and maintained in glucose-free DMEM. The cells were then placed into a hypoxic incubator (Binder, CB-210 hypoxia workstation) under 1% O2, 5% CO2, and 94% N2 for 8 h at 37° C to mimic OGD injury. The culture medium was then replaced with glucose-containing DMEM, and the cells recovered under normoxic conditions (37° C, 5% CO2) for 12 h (OGD restoration) as described previously [58]. Cells in the control groups without OGD were washed twice with PBS and maintained in DMEM without oxygen deprivation.
Drug treatment and in vitro cell viability assay
The Cell Counting Kit (CCK)-8 assay (Dojindo Laboratories, Kumamoto, Japan) was used to assess cell viability. Briefly, HUVECs were seeded in 96-well plates containing DMEM containing 10% FBS. The TGF-β inhibitor LY2109761 (S2704, Selleckchem, Houston, TX, USA) was reconstituted in DMSO and stored at -80° C. First, HUVECs at the beginning of the OGD period were treated with LY2109761 at different concentrations (0, 0.5, 1, 1.5, and 2 μM) [45] and Coicis Semen at different concentrations (10, 400, 500, 600, and 700 μM) [24] after OGD/RX to choose the appropriate doses. Then, the medium was removed, and 10 μl of CCK-8 solution was added to each well. After 2 h of incubation at 37° C, the absorbance at 450 nm was measured using an automatic microplate reader (PerkinElmer Victor 1420, Alburgh, VT, United States).
Western blot analysis
Total protein was extracted from HUVECs and ipsilateral brain tissue harvested after MCAO. Then, the samples were homogenized in cold RIPA buffer (C1053, Applygen, Beijing, China), and protease inhibitor cocktail (G2006, Servicebio, Wuhan, China) was added. The homogenates were centrifuged at 4° C at 10,000 × g for 30 min, and the supernatants were then harvested. The protein content was determined with a BCA kit (G2026, Servicebio, Wuhan, China). Protein samples (20 μL/lane) were separated by electrophoresis on 4-15% sodium dodecyl sulfate-polyacrylamide gels and then transferred to PVDF membranes (Millipore, Billerica, MA, United States). The membranes were then blocked in 5% nonfat milk with PBS/0.1% Tween for 1 h, followed by incubation overnight with anti-ZO-1 (1:1,000, ab96587, Abcam, Cambridge, United Kingdom), anti-Occludin (1:1,000, ab216327, Abcam, Cambridge, United Kingdom), anti-TGF-β1 (1:1,000, ab92486, Abcam, Cambridge, United Kingdom), anti-ALK-1 (1:1,000, ab108207, Abcam, Cambridge, United Kingdom), anti-Smad1/5 (1:500, ab66737, Abcam, Cambridge, United Kingdom), anti-phosphorylated (p-)Smad1/5 (1:500, #9516, Cell Signaling Technology, Boston, MA, United States), anti-VEGF (1:1,000, #2479, Cell Signaling Technology, Boston, MA, United States), and anti-CD31 (1:1,000, ab28364, Abcam, Cambridge, United Kingdom) antibodies at 4° C. After washing with PBS/0.1% Tween, the membranes were incubated with HRP-labeled secondary antibody (1:10,000, ab6721 for anti-rabbit, ab6728 for anti-mouse, Abcam, Cambridge, United Kingdom) at room temperature for 1 h. Then, ECL-A buffer and ECL-B buffer (Servicebio, Wuhan, China) were used for imaging. Images were acquired with a ChemiDoc imaging system (Bio-Rad). The relative band intensities were calculated using Quantity One v4.6.2 software (Bio-Rad Laboratories, Hercules, CA, United States) and then normalized to the control, GAPDH. All of the above experiments were performed three times.
Statistical analysis
Data are presented as the mean ± SD. Differences were considered to be significant at P < 0.05. The normality of the distribution of all data included in these studies was analyzed using the Shapiro-Wilk test. The F-test was used to evaluate equal variances. Then, we used one-way analysis of variance (ANOVA) with Tukey's post hoc test and two-way repeated-measures ANOVA with Sidak post hoc test, followed by the least significant difference test to analyze differences using GraphPad Prism 5.0 (GraphPad Software for Science, San Diego, CA). All assessments were performed by blinded observers.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request in compliance with ethical standards.
Ethics statement
The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University and performed in compliance with the ARRIVE guidelines.
Chaoliang Tang and Haiyan Lyu were involved in the design and execution of experiments, data analysis, and manuscript writing; Jin Du and Guobing Yin were involved in the designing of the study, the execution of experiments and data analysis and manuscript writing; Yida Hu, Si Shi, Jiazhen Jiang, Xiaoyan Song, Zhetao Zhang, and Zeyuan Wei were all involved in the execution of experiments and data analysis; All authors provided final approval of the version to be submitted.
The authors declare that they have no conflicts of interests.
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81801175 to Chaoliang Tang), the China Postdoctoral Science Foundation (Grant No. 2019M662179 to Chaoliang Tang), the Anhui Province Postdoctoral Science Foundation (Grant No. 2019B324 to Chaoliang Tang), and the Fundamental Research Funds for the Central Universities (Grant No. WK9110000044 to Chaoliang Tang).