Genomic distribution of hypermethylated and hypomethylated DMPs
To explore the abnormal methylation status of the entire genome, we conducted in-depth studies on the early diagnosis and prognostic evaluation of COAD patients (Figure 1). First, we performed CpG site expression profiling analysis between COAD tumor samples (N = 25) and paired normal samples (N = 25) from The Cancer Genome Atlas (TCGA) cohort. Then, 13716 Hyper-DMPs and 11403 Hypo-DMPs were obtained in these included cohorts. Specifically, unsupervised cluster analysis distinguished these Hyper-DMPs and Hypo-DMPs in 25 paired COAD and normal samples from TCGA (Figure 2A). When we assessed the locations of these Hyper-DMPs and Hypo-DMPs among genomic region types, we observed that Hyper-DMPs were most abundant in Island regions (39%), whereas Hypo-DMPs were mainly distributed in Open sea regions (42%) (Figure 2B). We also determined the enrichment of the DMPs by calculating the ratio of Hyper-DMPs to Hypo-DMPs in each region. The results indicated that Hyper-DMPs were enriched in Island regions (66%; Hyper/Hypo = 5421/2689), whereas Hypo-DMPs occurred more frequently in Open sea regions (58%; Hypo/Hyper = 4882/3588).
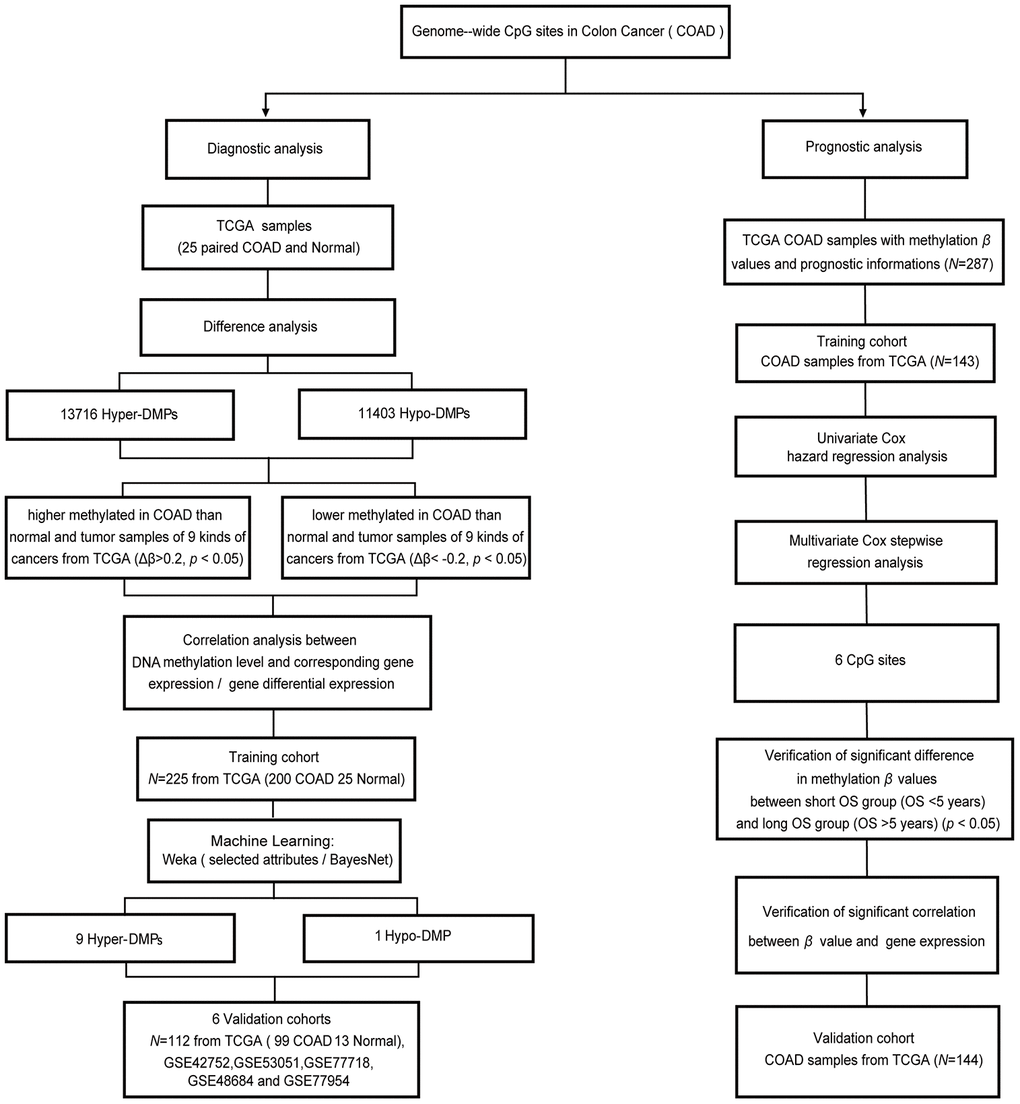
Figure 1. Workflow diagram for biomarker screening and model construction. The DNA methylation levels of genome-wide CpG sites were used to screen biomarkers and construct diagnostic and prognostic models of COAD. Left side: diagnostic biomarker selection and COAD-specific diagnostic model construction. Conditional screening and machine learning using the selected attributes and BayesNet functions of WEKA were performed to obtain the final nine Hyper-DMPs and one Hypo-DMP as potential biomarkers in the training cohort from TCGA (including 200 COAD and 25 normal samples). BayesNet was used to evaluate the COAD-specific diagnostic model based on these DMPs in the validation cohort from TCGA (including 99 COAD and 13 normal samples) and five independent GEO cohorts (GSE42752, GSE53051, GSE77718, GSE48684 and GSE77954). Right side: prognostic biomarker selection and COAD prognostic model construction. Univariate Cox hazard regression analysis and multivariate Cox stepwise regression analysis were applied to 143 TCGA COAD samples as the training cohort to obtain six CpG sites as potential biomarkers. The prognostic model based on these six CpG sites was evaluated using 144 TCGA COAD samples as the validation cohort.
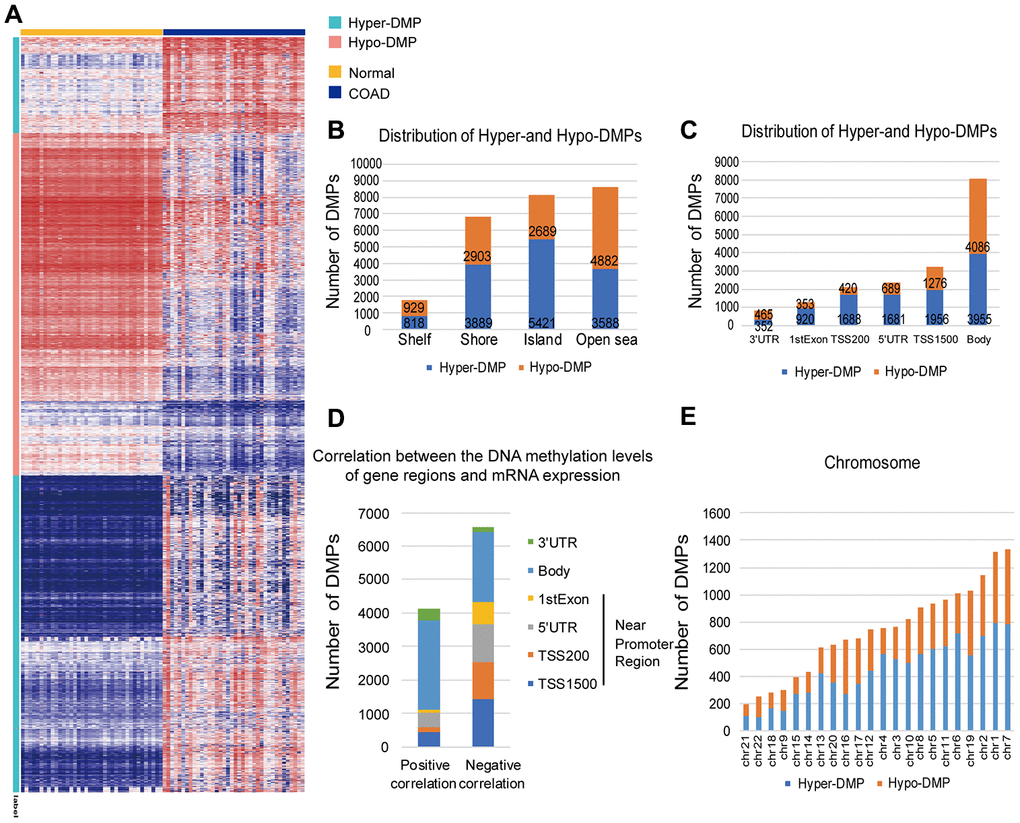
Figure 2. Distribution of DMPs. (A) Unsupervised hierarchical clustering and heat map display of the methylation levels of the Hyper- and Hypo-DMPs in 25 paired COAD and normal samples from TCGA. (B) The distribution of Hyper-DMPs and Hypo-DMPs in different genomic region types. Island, a CpG site located within a CpG island; Shore, a CpG site located < 2 kilobases from a CpG island; Shelf, a CpG site located > 2 kilobases from a CpG island; Open sea, a CpG site not in an island or annotated gene. (C) The numbers and ratios of Hyper-DMPs and Hypo-DMPs according to their distance from the promoter. TSS1500, 200-1500 base pairs upstream of the transcription start site; TSS200, 200 base pairs upstream of the transcription start site; 5′UTR, 5′ untranslated region; 1st Exon, exon 1; 3′UTR, 3′ untranslated region. (D) The positional distribution (in terms of promoter distance) of the DMPs in which the methylation level correlated positively or negatively with the expression of the corresponding gene (FDR < 0.05). (E) Chromosome distribution of Hyper-DMPs and Hypo-DMPs. Chr: chromosome.
More importantly, Hyper-DMPs were mainly located near promoter regions, including TSS1500 (the region 200 to 1500 nucleotides upstream of the transcription start site), TSS200 (the region from the transcription start site to 200 nucleotides upstream of the transcription start site), the 5′ untranslated region (UTR) and the 1st Exon (Figure 2C). However, Hypo-DMPs were mostly enriched in the Body and the 3′UTR, which occupied a large percentage of the regions, genome-wide. The DMP distribution ratio also indicated that proximal promoter regions were mainly hypermethylated (69%; hyper/hypo = 6245/2738), while the proportions of Hyper- and Hypo-DMPs in the Body and 3′UTR were almost equal (51%; hypo/hyper = 4551/4307). Notably, both Hyper-DMPs and Hypo-DMPs occupied a large proportion of the whole genome, about 3.42%.
Next, we calculated Pearson correlation coefficients to determine the correlation between the DNA methylation of the DMPs and the expression of their corresponding genes (Figure 2D). Among the 17112 DMPs for which both the DNA methylation levels and the corresponding mRNA expression profiles were available, the methylation levels of 6565 Hyper-DMPs and 4112 Hypo-DMPs were significantly associated with the mRNA levels of the corresponding genes (|r| > 0.1, false discovery rate [FDR] < 0.05). When we analyzed the distance between these DMPs and promoter regions, we found that DMPs in or near promoter regions (i.e., in the 1st Exon, 5′ UTR, TSS200 or TSS1500) were negatively associated with mRNA expression, whereas those outside promoter regions (i.e., in the Body or 3′ UTR) were positively associated with gene expression. Moreover, the DMPs had a higher distribution frequency on chromosomes 7 and 1 than on the other autosomes (Figure 2E). Since the DMPs that significantly altered the expression of their corresponding genes were not limited to promoter regions, we screened the whole genome for potential biomarkers of COAD and constructed a diagnostic prediction model based on the genome-wide Hyper- and Hypo-DMPs.
Identification of COAD-specific methylation biomarkers and construction of a COAD-specific diagnostic model
Next, in order to construct a diagnostic model to distinguish COAD tumor tissues from normal intestinal epithelial tissues and the tumor tissues of nine other cancer types (BLCA, BRCA, CESC, glioblastoma [GBM], head and neck cancer [HNSC], liver cancer [LIHC], lung adenocarcinoma [LUAD], lung squamous cell carcinoma [LUSC] and endometrial cancer [UCEC]), we performed conditional screening and machine learning studies based on the genome-wide DMPs obtained from TCGA above (13716 Hyper-DMPs and 11403 Hypo-DMPs). For the conditional screening, we determined the average β values (a measure of CpG site methylation) of these Hyper-/Hypo-DMPs in all the samples for the nine cancer types in TCGA. Then, we selected DMPs based on an average methylation level difference of at least 0.2 units in COAD. After screening under the above conditions, we obtained 17 Hyper-DMPs and 8 Hypo-DMPs as candidate biomarkers. Further analysis revealed that these 17 Hyper-DMPs and 8 Hypo-DMPs were among the 6565 Hyper-DMPs and 4112 Hypo-DMPs that were significantly associated with the expression of their corresponding genes (log2 |fold change| > 1, FDR < 0.05).
For machine learning, two-thirds of the total tumor and normal samples from the COAD cohort of TCGA (200 COAD and 25 normal samples) were randomly set as the training cohort, while the remaining one-third of the total samples (99 COAD and 13 normal samples) were used as the validation cohort. The β values of the 17 Hyper-DMPs and 8 Hypo-DMPs in the training cohort were input into WEKA, and the selected attributes function of WEKA was used to filter these candidate biomarkers. As potential diagnostic biomarkers, nine Hyper-DMPs (cg26036626, cg03882585, cg08130988, cg16733654, cg12587766, cg08808128, cg13004587, cg05038216 and cg09746736) and one Hypo-DMP (cg26718707) were selected to construct a COAD-specific diagnostic model (Table 1). Finally, based on the nine Hyper-DMPs and one Hypo-DMP, we constructed a COAD-specific diagnostic model with BayesNet [23].
Table 1. Characteristics of 9 Hyper-DMPs and 1 Hypo-DMP in the COAD-specific diagnostic model.
| Biomarkers | Ref Gene | Chromosome | Start | End | CGI Coordinate | Feature | CGI | FDR | Type |
| cg26036626 | FBLIM1 | chr1 | 15759102 | 15759103 | 15758576-15759367 | 5'UTR | Island | 1.79e-15 | Hyper-DMP |
| cg03882585 | SYNE1 | chr6 | 152636775 | 152636776 | 152636675-152637337 | 5'UTR | Island | 1.29e-05 | Hyper-DMP |
| cg08130988 | EFEMP1 | chr2 | 55923790 | 55923791 | 55923205-55923813 | 1st Exon | Island | 6.49e-05 | Hyper-DMP |
| cg16733654 | PTPRS | chr19 | 5293072 | 5293073 | 5292760-5294200 | 5'UTR | Island | 7.65e-09 | Hyper-DMP |
| cg12587766 | LIFR | chr5 | 38556333 | 38556334 | 38556120-38557461 | 1st Exon | Island | 2.19e-11 | Hyper-DMP |
| cg08808128 | CLIP4 | chr2 | 29115566 | 29115567 | 29115117-29116043 | 1st Exon | Island | 4.48e-09 | Hyper-DMP |
| cg13004587 | SCGB3A1 | chr5 | 180590349 | 180590350 | 180590099-180592062 | Body | Island | 0.0448 | Hyper-DMP |
| cg05038216 | CLIP4 | chr2 | 29116225 | 29116226 | 29115117-29116043 | 5'UTR | Shore | 1.82e-09 | Hyper-DMP |
| cg09746736 | SLC6A2 | chr16 | 55656218 | 55656219 | 55655686-55656983 | TSS 1500 | Island | 2.38e-08 | Hyper-DMP |
| cg26718707 | DIP2C | chr10 | 472430 | 472431 | 472252-472531 | Body | Island | 0.0107 | Hypo-DMP |
| CGI: CpG island |
The average β values of the nine Hyper-DMPs and one Hypo-DMP selected for our diagnostic model in all the COAD tissues, normal tissues and nine types of cancerous tissues from TCGA are visualized in Figure 3A. We performed an unsupervised cluster analysis to evaluate these β values (Figure 3B), and found that they were clearly divided into four clusters. The COAD tumor samples were significantly differentiated from all the normal samples and the tumor samples from the nine other cancer types based on the nine Hyper-DMPs and one Hypo-DMP.
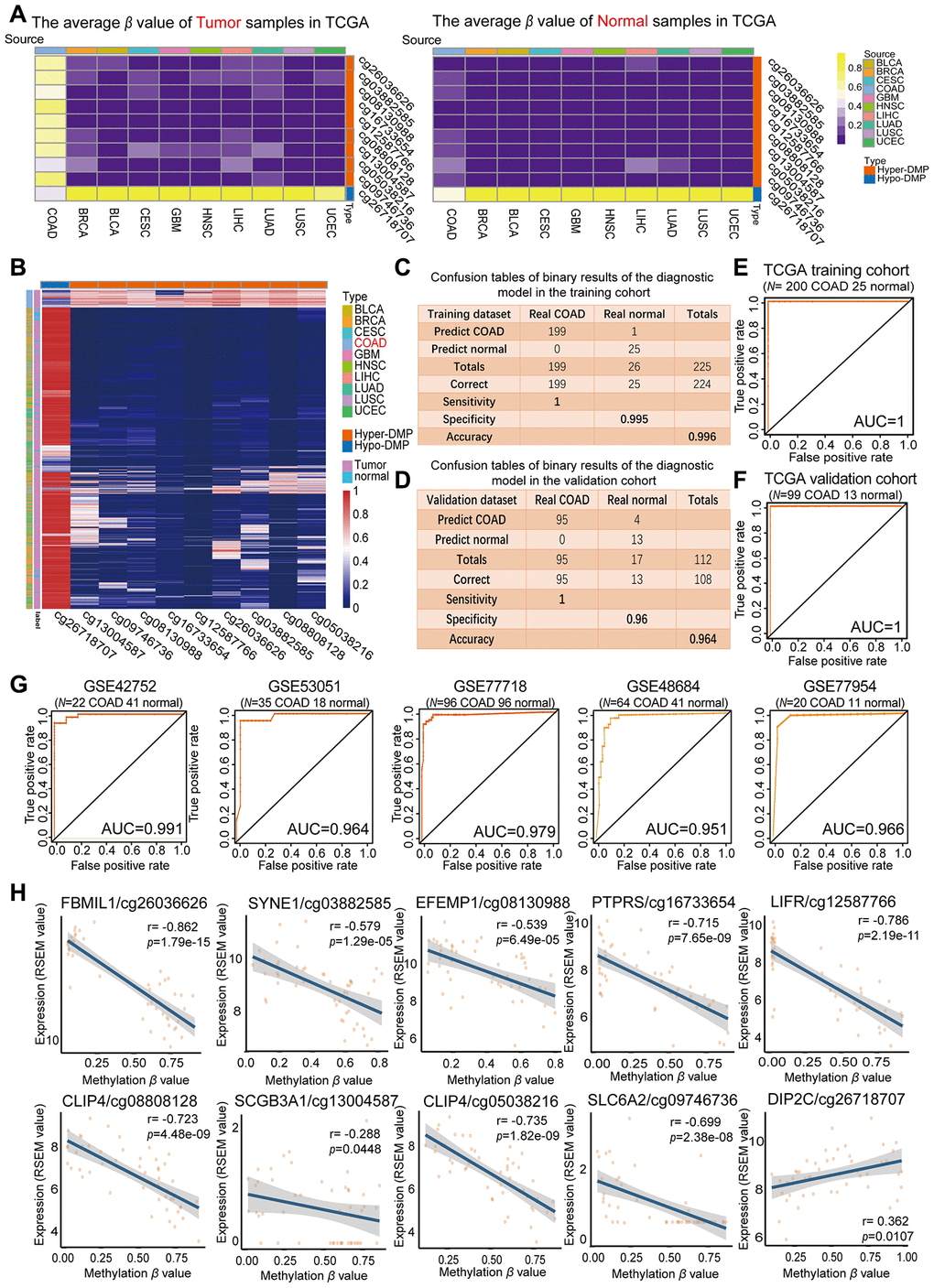
Figure 3. Evaluation of the COAD-specific diagnostic biomarkers and diagnostic model. (A) Heat maps of the average methylation levels of the nine Hyper-DMPs and one Hypo-DMP in all the samples from 10 cancer types. The legend on the right marks the source and CpG type. The picture on the left represents the tumor samples in TCGA, while the picture on the right represents the normal samples in TCGA. (B) Unsupervised hierarchical clustering of the methylation levels of the nine Hyper-DMPs and one Hypo-DMP in all the samples from 10 cancer types. The legend on the right marks the source and CpG type. (C–F) Confusion tables (C, E) and corresponding ROC curves (D, F) for the binary results of the COAD-specific diagnostic model in the training cohort (N = 225) and the validation cohort (N = 112) from TCGA. (G) ROC curves of the COAD-specific diagnostic model in five GEO COAD validation cohorts (GSE42752, GSE53051, GSE77718, GSE48684 and GSE77954, which included 22 COAD and 41 normal samples, 35 COAD and 18 normal samples, 96 paired COAD and normal samples, 64 COAD and 41 normal samples, and 20 COAD and 11 normal samples, respectively). (H) The correlation between the DMP methylation level and the expression of the corresponding gene for each diagnostic biomarker, determined through Pearson correlation tests (r > 0.2, FDR < 0.05). Gene expression is presented as the RSEM normalized count converted by log2 (x + 1).
Subsequently, we used the COAD-specific diagnostic model to train the training cohort (including 200 COAD and 25 normal samples from TCGA) in WEKA. Using BayesNet, we determined that the COAD-specific diagnostic model had a sensitivity of 100%, specificity of 99.5% and accuracy of 99.6% in the training cohort (Figure 3C). In the validation cohort (99 COAD and 13 normal samples from TCGA), the diagnostic model had a sensitivity of 100%, specificity of 96% and accuracy of 96.4% (Figure 3D). Therefore, our COAD-specific diagnostic model was confirmed to perfectly distinguish between COAD and normal samples in the training cohort (AUC = 1) (Figure 3E) and the validation cohort (AUC = 1) from TCGA (Figure 3F).
To demonstrate the versatility of our diagnostic model, we conducted a population heterogeneity analysis using the validation cohort from TCGA, which included samples from 4 Asian, 25 black or African American and 73 white patients. The sensitivity, specificity and accuracy are shown in Table 2. Our diagnostic model exhibited no significant population heterogeneity, suggesting that it can be applied to people of different races. In addition, we used the five independent GEO COAD cohorts mentioned above (GSE42752, GSE53051, GSE77718, GSE48684 and GSE77954) as validation cohorts. In receiver operating characteristic (ROC) analyses, the AUCs of these five cohorts were 0.991, 0.964, 0.979, 0.951 and 0.966, respectively (Figure 3G). These results further illustrated the reproducibility and stability of our COAD-specific diagnostic model.
Table 2. The stratification analysis of the sensitivity, specificity, and accuracy from different races including 4 Asian, 25 Black or African American, and 73 White upon TCGA validation cohort.
| Race | Asian | Black or African American | White |
| Sensitivity | 0 | 1 | 1 |
| Specificity | 1 | 0.913 | 0.97 |
| Accuracy | 1 | 0.92 | 0.973 |
We also analyzed the correlation between the DMP methylation level and the expression of the corresponding genes for the nine Hyper-DMPs and the one Hypo-DMP in our diagnostic model. The Hypo-DMP (cg26718707) corresponded to DIP2C, while the nine Hyper-DMPs corresponded to eight genes: FBLIM1 (cg26036626), SYNE1 (cg03882585), EFEMP1 (cg08130988), PTPRS (cg16733654), LIFR (cg12587766), CLIP4 (cg08808128 and cg05038216), SCGB3A1 (cg13004587) and SLC6A2 (cg09746736). The results of the correlation analysis are shown in Figure 3H and Supplementary Table 1. The expression of DIP2C correlated positively with the methylation level of the Hypo-DMP (r > 0.1, FDR < 0.05), and the expression of the other eight genes correlated negatively with the methylation levels of the corresponding nine Hyper-DMPs (r < -0.1, FDR < 0.05).
Next, using the five independent GEO cohorts, we compared our COAD-specific diagnostic model with three previously reported methylation-based diagnostic models: a Bayesian model including four CpG sites from Azuara et al. [24], a logistic regression model including five CpG sites from Beggs et al. [25] and a logistic regression model including 12 CpG sites from Naumov et al. [26] (Figure 4A). As expected, our model exhibited better sensitivity, specificity and accuracy than the three previously reported diagnostic models in most cases for the five GEO COAD cohorts. We also compared our diagnostic model with these three models in terms of its ability to distinguish COAD from normal tissues and nine other types of cancerous tissues. For this purpose, we divided all the samples from the five GEO COAD cohorts and nine various TCGA cancerous cohorts into a tumor group and a normal group, and we calculated the proportion of samples that were predicted to be COAD among all the samples (Figure 4B, Table 3). In the cohorts from the nine different types of cancers, the ideal proportion would have been 0. When we tested our COAD-specific diagnostic model, almost none of the normal intestinal epithelial samples or the tumor tissues from the nine other cancer types were predicted as COAD (0-5%, median: 0%). However, when we tested the three previously reported diagnostic models, 0-97.7% of the normal tissues (median: 0%, 33.3% and 0%, respectively) and 20.2-98.7% of the tumor tissues from the nine other cancer types (median: 45.4%, 93.5% and 75.2%, respectively) were predicted as COAD. Therefore, our COAD-specific diagnostic model based on nine Hyper-DMPs and one Hypo-DMP not only distinguished COAD from normal samples, but also compensated for the deficiencies of previous COAD diagnostic models that could not differentiate COAD from nine other cancer types.
![Performance comparison of diagnostic models and enrichment analysis of the corresponding genes. (A) Table displaying the classification performance of different methylation models for COAD and normal tissues in five independent GEO cohorts (GSE42752, GSE53051, GSE77718, GSE48684 and GSE77954). In addition, Azuara et al. [24] (Article 1) reported four CpG sites as diagnostic biomarkers for COAD, and the methylation values for each of them were available in the COAD cohort from TCGA; Beggs et al. [25] (Article 2) reported six CpG sites as diagnostic biomarkers for COAD, and the methylation values for five of them were available in the COAD cohort from TCGA; and Naumov et al. [26] (Article 3) reported 14 CpG sites as diagnostic biomarkers for COAD, and the methylation values for 12 of them were available in the COAD cohort from TCGA. (B) Heat map comparing our diagnostic model with the previous methylation models. Rows are labeled with the different sources of methylation data. The legend indicates that the range is 0-1. The color represents the percentage of the total samples predicted to be COAD. In the cohorts for the nine different cancer types, the ideal results should be 0. (C) Predicted protein interaction network of the genes corresponding to the COAD-specific diagnostic biomarkers. Version 11.0 of the STRING protein database was used. The different line colors represent different kinds of correlations between the proteins corresponding to the model (dark blue for coexistence, black for co-expression, pink for an experiment, light blue for a database, green for text mining, and purple for homology). The red genes are the corresponding genes of the diagnostic biomarkers. Note that CLIP4 is the corresponding gene for both cg08808128 and cg05038216. (D, E) KEGG (D) and GO (E) enrichment analysis results from the STRING protein database. All seven results are shown for the KEGG enrichment analysis, and the top 10 results are shown for the GO enrichment analysis, with p-values arranged from large to small. In the KEGG enrichment graph (D), the X-axis represents the Rich factor, indicating the degree of enrichment (Rich factor = observed gene counts/background gene counts), and the Y-axis represents the enriched KEGG terms. The color represents the -log10 (p-value), and the size of the dot represents the number of genes. In the GO enrichment graph (E), the GO term indicates the GO enrichment pathway.](/article/103874/figure/f4/large)
Figure 4. Performance comparison of diagnostic models and enrichment analysis of the corresponding genes. (A) Table displaying the classification performance of different methylation models for COAD and normal tissues in five independent GEO cohorts (GSE42752, GSE53051, GSE77718, GSE48684 and GSE77954). In addition, Azuara et al. [24] (Article 1) reported four CpG sites as diagnostic biomarkers for COAD, and the methylation values for each of them were available in the COAD cohort from TCGA; Beggs et al. [25] (Article 2) reported six CpG sites as diagnostic biomarkers for COAD, and the methylation values for five of them were available in the COAD cohort from TCGA; and Naumov et al. [26] (Article 3) reported 14 CpG sites as diagnostic biomarkers for COAD, and the methylation values for 12 of them were available in the COAD cohort from TCGA. (B) Heat map comparing our diagnostic model with the previous methylation models. Rows are labeled with the different sources of methylation data. The legend indicates that the range is 0-1. The color represents the percentage of the total samples predicted to be COAD. In the cohorts for the nine different cancer types, the ideal results should be 0. (C) Predicted protein interaction network of the genes corresponding to the COAD-specific diagnostic biomarkers. Version 11.0 of the STRING protein database was used. The different line colors represent different kinds of correlations between the proteins corresponding to the model (dark blue for coexistence, black for co-expression, pink for an experiment, light blue for a database, green for text mining, and purple for homology). The red genes are the corresponding genes of the diagnostic biomarkers. Note that CLIP4 is the corresponding gene for both cg08808128 and cg05038216. (D, E) KEGG (D) and GO (E) enrichment analysis results from the STRING protein database. All seven results are shown for the KEGG enrichment analysis, and the top 10 results are shown for the GO enrichment analysis, with p-values arranged from large to small. In the KEGG enrichment graph (D), the X-axis represents the Rich factor, indicating the degree of enrichment (Rich factor = observed gene counts/background gene counts), and the Y-axis represents the enriched KEGG terms. The color represents the -log10 (p-value), and the size of the dot represents the number of genes. In the GO enrichment graph (E), the GO term indicates the GO enrichment pathway.
Table 3. Comparison of the performance of different methylation models in all samples of 9 kinds of cancers.
| Accuracy | BLCAC | BLCAN | BRCAC | BRCAN | CESCC | CESCN | GBMC | GBMN | HNSCC | HNSCN |
| 10 DMPs biomarker | 0.022 | 0 | 0.05 | 0 | 0.05 | 0 | 0 | 0 | 0.02 | 0 |
| 4 CpGs of Article 1 | 0.593 | 0.095 | 0.584 | 0.01 | 0.663 | 0 | 0.327 | 0 | 0.742 | 0.02 |
| 5 CpGs of Article 2 | 0.939 | 0.333 | 0.92 | 0.388 | 0.977 | 0 | 0.928 | 0 | 0.977 | 0.977 |
| 12 CpGs of Article 3 | 0.872 | 0.19 | 0.749 | 0.143 | 0.625 | 0 | 0.242 | 0 | 0.87 | 0.02 |
| Accuracy | LIHCC | LIHCN | LUADC | LUADN | LUSCC | LUSCN | UCECC | UCECN | | |
| 10 DMPs biomarker | 0.03 | 0 | 0.01 | 0 | 0 | 0 | 0.04 | 0 | | |
| 4 CpGs of Article 1 | 0.413 | 0 | 0.454 | 0 | 0.202 | 0 | 0.348 | 0 | | |
| 5 CpGs of Article 2 | 0.871 | 0.08 | 0.987 | 1 | 0.935 | 0.93 | 0.843 | 0.043 | | |
| 12 CpGs of Article 3 | 0.375 | 0 | 0.752 | 0 | 0.815 | 0 | 0.944 | 0.043 | | |
| The vertical axis shows the different sources of the methylation models. The horizontal axis shows the tumor and normal methylation cohorts from 9 kinds of cancer. Affix C represents the tumor samples of the methylation data sets. Affix N represents the normal sample of the methylation cohorts. The numbers represent the percentage of samples predicted to be COAD in the total samples. The cohorts in the table are from 9 kinds of cancers, and the ideal result should be 0. Azuara D et al. [24] (Article 1)reported 4 CpG sites as diagnostic markers for COAD, and 4 of them had methylation values in the TCGA COAD cohort; Beggs AD et al. [25] (Article 2)report 6 CpG sites as diagnostic markers for COAD, and 5 of them had methylation values in the TCGA COAD cohort; Naumov VA et al. [26] (Article 3)reported 14 CpG sites as diagnostic markers for COAD, and 12 of them had methylation values in the TCGA COAD cohort. |
Then, we used the STRING database to construct a protein-protein interaction network for the nine genes corresponding to the nine Hyper-DMPs and the one Hypo-DMP (Figure 4C). We also performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses on these genes (Figure 4D and 4E). We found that the nine genes were involved in important signaling pathways of tumorigenesis and development, such as Salmonella infection, Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling, focal adhesion, proteoglycans in cancer, cytokine-cytokine receptor interactions, etc. All the results of the KEGG pathway analysis and the top 10 results of the GO analysis are shown in Supplementary Tables 2 and 3.
The above results demonstrated that our COAD-specific diagnostic model could accurately and precisely distinguish COAD tissues from normal intestinal epithelial samples and tumor samples from nine cancer types, and that the nine Hyper-DMPs and one Hypo-DMP included in this model may be potential biomarkers for the early prediction and specific diagnosis of COAD.
Identification of prognostic biomarkers of COAD and construction of a combined COAD prognostic model
A total of 287 COAD tissue samples in the cohort from TCGA had both methylated β values and corresponding prognostic information. The distribution and corresponding demographic characteristics of these patients are summarized in Table 4. The patients were divided into a training cohort (N = 143) and a validation cohort (N = 144). The training cohort was used to obtain prognostic biomarkers and construct a COAD prognostic model, while the validation cohort was used to test the COAD prognostic model. Univariate Cox hazard regression analysis of the training cohort revealed 64 CpG sites that correlated significantly with the OS of COAD patients (FDR < 0.05); thus, these CpG sites were identified as candidate prognostic biomarkers. Multivariate Cox stepwise regression analysis was applied to these 64 CpG sites, and six sites (cg00177496, cg01963906, cg05165940, cg12921795, cg19414598 and cg25783173) were included in our final hazard ratio model, which was constructed as a combined COAD prognostic model for OS prediction (Table 5). The six CpG sites from our COAD prognostic model were found to correspond to BDH1 (cg00177496), SYTL1 (cg01963906), SATB2 (cg05165940), WDR20 (cg12921795), DMC1 (cg19414598) and ZNF35 (cg25783173) (Figure 5A and Supplementary Table 4). The expression of SYTL1 correlated positively with the methylation level of cg01963906 (r > 0.1, FDR < 0.05), and the expression of the other five genes correlated negatively with the methylation levels of the remaining CpG sites (r < -0.1, FDR < 0.05).
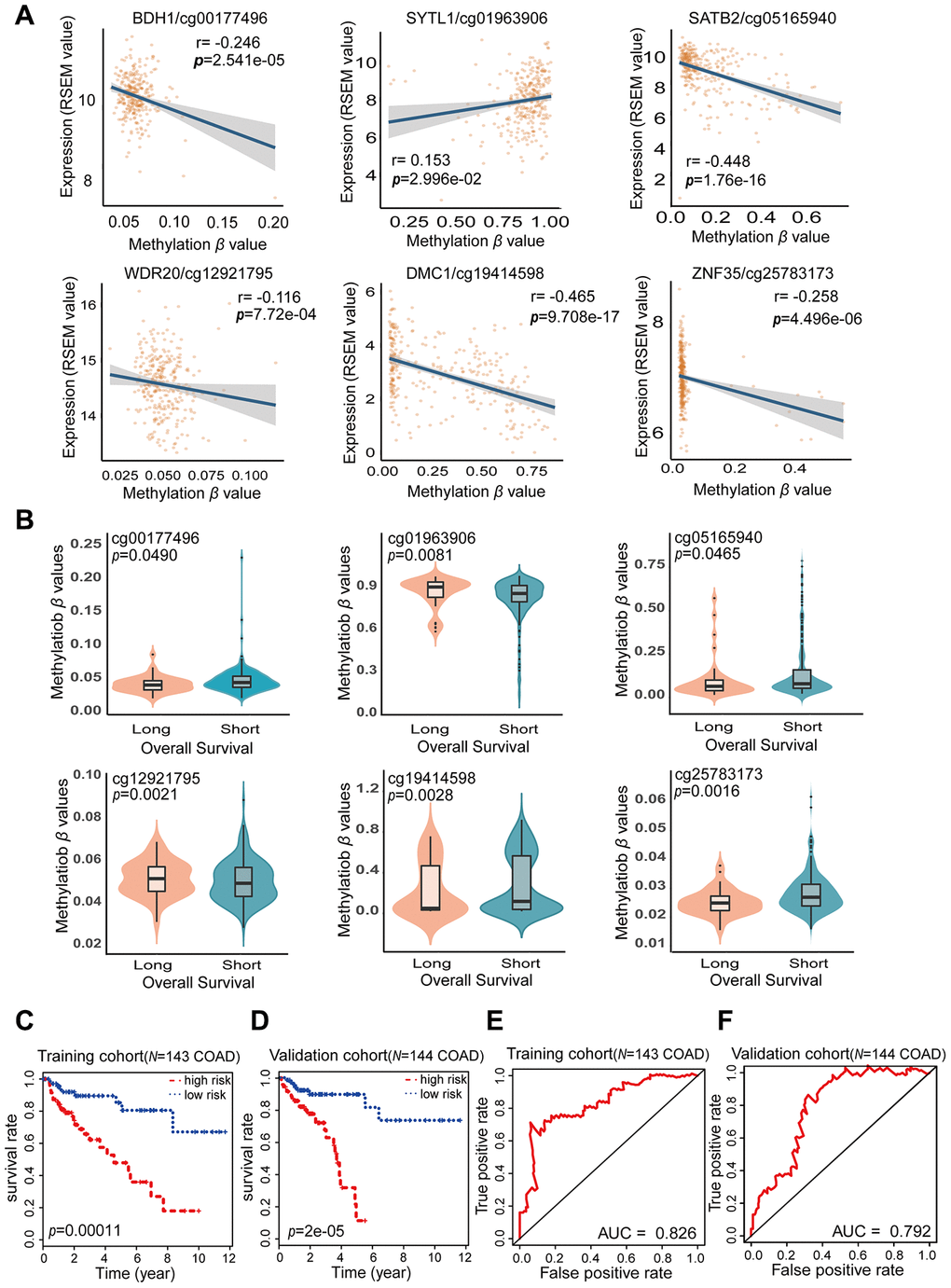
Figure 5. Characteristics of the potential prognostic biomarkers and evaluation of the combined prognostic model based on six CpG sites. (A) The correlations between the methylation β levels of the prognostic biomarkers and the expression of the corresponding genes were evaluated with Pearson correlation tests. Gene expression is presented as the RSEM normalized count converted by log2 (x + 1). (B) Violin plots of the methylation β values for patients with longer (> 5 years) and shorter (< 5 years) OS in the training cohort, with the median in the centerline. A Wilcoxon test was used to determine the difference between the two groups. The corresponding CpG sites, cor-values and p-values are shown at the top of the plot. (C, D) Kaplan-Meier analysis was performed on the OS of high-risk and low-risk patients using our prognostic model in the training (N = 143) (C) and validation (N = 144) (D) cohorts from TCGA. The difference in OS between the two groups was determined with a log-rank test. Higher risk scores were associated with significantly poorer OS. Patients were divided into low-risk and high-risk groups using the median risk score as the cut-off. (E, F) ROC curves showing the sensitivity and specificity of the prognostic model in predicting patients’ OS in the training (N = 143) (E) and validation (N = 144) (F) cohorts from TCGA.
Table 4. Clinicopathological characteristics of COAD patients from the TCGA database.
| Characteristics | Patients |
| Total (N = 287) | Training cohort (N = 143) | Validation cohort (N = 144) |
| No | % | No | % | No | % |
| Age | | | | | | |
| ≤64 | 130 | 45.30 | 58 | 40.56 | 72 | 50 |
| >64 | 157 | 54.70 | 85 | 59.44 | 72 | 50 |
| Histological type | | | | | | |
| Colon Adenocarcinoma | 246 | 85.71 | 119 | 83.22 | 127 | 88.19 |
| Colon Mucinous Adenocarcinoma | 38 | 13.24 | 22 | 15.38 | 16 | 11.11 |
| Unknown | 3 | 1.05 | 2 | 1.40 | 1 | 0.70 |
| Pathologic M | | | | | | |
| M0 | 195 | 67.94 | 102 | 71.33 | 93 | 64.58 |
| M1 | 40 | 13.94 | 21 | 14.69 | 19 | 13.19 |
| MX | 49 | 17.07 | 19 | 13.29 | 30 | 20.83 |
| Unknown | 3 | 1.05 | 1 | 0.07 | 2 | 1.39 |
| Pathologic N | | | | | | |
| N0 | 166 | 57.84 | 73 | 51.05 | 93 | 64.58 |
| N1 | 73 | 25.44 | 46 | 32.17 | 27 | 18.75 |
| N2 | 48 | 16.72 | 24 | 1.40 | 24 | 16.67 |
| Pathologic T | | | | | | |
| T1 | 7 | 2.44 | 2 | 1.39 | 5 | 3.47 |
| T2 | 42 | 14.63 | 17 | 11.89 | 25 | 17.36 |
| T3 | 199 | 69.34 | 105 | 73.43 | 94 | 65.28 |
| T4 | 38 | 13.24 | 19 | 13.29 | 19 | 13.19 |
| Unknown | 1 | 0.35 | 0 | 0 | 1 | 0.70 |
| Gender | | | | | | |
| Female | 134 | 46.69 | 70 | 48.95 | 64 | 44.44 |
| Male | 153 | 53.31 | 73 | 51.05 | 80 | 55.56 |
| Race | | | | | | |
| American Indian or Alaska Native | 1 | 0.35 | 1 | 0.70 | 0 | 0 |
| Asian | 11 | 3.83 | 4 | 2.80 | 7 | 4.86 |
| Black or African American | 57 | 19.86 | 20 | 13.98 | 37 | 25.70 |
| White | 201 | 70.04 | 108 | 75.52 | 93 | 64.58 |
| Unknown | 17 | 5.92 | 10 | 7.00 | 7 | 4.86 |
| Tumor stage | | | | | | |
| Stage I | 43 | 14.98 | 16 | 11.19 | 27 | 18.75 |
| Stage II | 110 | 38.33 | 52 | 36.36 | 58 | 40.28 |
| Stage III | 84 | 29.27 | 51 | 14.69 | 33 | 22.92 |
| Stage IV | 40 | 13.94 | 21 | 2.10 | 19 | 13.19 |
| Unknown | 10 | 3.48 | 3 | 27.27 | 7 | 4.86 |
| Lymphatic invasion | | | | | | |
| Yes | 76 | 26.48 | 39 | 27.27 | 37 | 25.69 |
| No | 175 | 60.98 | 88 | 61.54 | 87 | 60.42 |
| Unknown | 36 | 12.54 | 16 | 11.19 | 20 | 13.89 |
| Primary lymph node presentation assessment | | | | | | |
| Yes | 265 | 92.33 | 134 | 93.70 | 131 | 90.97 |
| No | 14 | 4.88 | 5 | 3.50 | 9 | 6.25 |
| Unknown | 8 | 2.79 | 4 | 2.80 | 4 | 2.78 |
| Vital status | | | | | | |
| Alive | 218 | 75.96 | 107 | 74.83 | 111 | 77.08 |
| Dead | 69 | 24.04 | 36 | 25.17 | 33 | 22.92 |
| Longest dimension | | | | | | |
| ≥2 | 43 | 14.98 | 27 | 18.88 | 16 | 11.11 |
| <2 | 175 | 60.98 | 90 | 62.94 | 85 | 59.03 |
| Unknown | 69 | 24.04 | 26 | 18.18 | 43 | 29.86 |
| Sample type | | | | | | |
| Metastatic | 1 | 0.35 | 0 | 0 | 1 | 0.69 |
| Primary Tumor | 285 | 99.30 | 142 | 99.30 | 143 | 99.31 |
| Recurrent Tumor | 1 | 0.35 | 1 | 0.70 | 0 | 0 |
| Lymph node examined count | | | | | | |
| ≥12 | 226 | 78.75 | 117 | 81.82 | 109 | 75.70 |
| <12 | 42 | 14.63 | 19 | 13.28 | 23 | 15.97 |
| Unknown | 19 | 6.62 | 7 | 4.90 | 12 | 8.33 |
Table 5. Characteristics of prognostic biomarkers and their coefficients in the combined COAD prognostic model.
| Biomarkers | Ref Gene | Coefficients | HR | CI (lower) | CI (upper) | SE | z value | CGI | FDR |
| cg00177496 | BDH1 | 38.52 | 5.3E+16 | 3.55E+02 | 8.02E+30 | 16.66 | 2.312 | Island | 2.541e-05 |
| cg01963906 | SYTL1 | -4.13 | 0.01608 | 1.86E-03 | 1.39E-01 | 1.102 | -3.75 | Island | 2.996e-02 |
| cg05165940 | SATB2 | 2.574 | 13.12 | 2.70E+00 | 6.38E+01 | 0.8072 | 3.189 | Island | 1.76e-16 |
| cg12921795 | WDR20 | -79.32 | 3.58E-35 | 2.16E-57 | 5.91E-13 | 26.1 | -3.039 | Island | 7.72e-04 |
| cg19414598 | DMC1 | 2.31 | 10.07 | 2.75E+00 | 3.68E+01 | 0.6616 | 3.491 | Island | 9.708e-17 |
| cg25783173 | ZNF35 | 6.061 | 429 | 1.60E+01 | 1.15E+04 | 1.677 | 3.614 | Island | 4.496e-06 |
| HR: Hazard Ratio; CI: 95.0% confidence interval; SE: standard errors of coefficients; z value: Wald z-statistic value. |
The risk score formula for our COAD prognostic model was based on the regression coefficients and methylation levels of the six CpG sites, as follows: risk score = (38.52 × cg00177496 β value) – (4.13 × cg01963906 β value) + (2.574 × cg05165940 β value) – (79.32 × cg12921795 β value) + (2.31 × cg19414598 β value) + (6.061 × cg25783173 β value). In the risk score formula, a positive coefficient for a CpG site (cg00177496, cg05165940, cg19414598 and cg25783173) indicates that hypermethylation of that site was associated with shorter OS in COAD patients. In contrast, a negative coefficient for a CpG site (cg01963906 and cg12921795) indicates that greater methylation of that site was associated with longer OS. Our COAD prognostic model revealed that there were significant differences in DNA methylation levels between patients with long-term (> 5 years) and short-term (< 5 years) survival (FDR < 0.05) (Figure 5B). Consistent with the results of the multivariate Cox stepwise regression analysis, the CpG sites with positive coefficients (cg00177496, cg05165940, cg19414598 and cg25783173) exhibited lower methylation levels in patients who survived long-term, while the CpG sites with negative coefficients (cg01963906 and cg12921795) exhibited higher methylation levels in patients who survived long-term. Thus, our combined COAD prognostic model based on six CpG sites successfully distinguished long-term from short-term surviving patients in the training cohort of 143 COAD samples from TCGA.
Based on the Cox regression analyses, risk scores were used as continuous variables in the training and validation cohorts. The risk scores obtained from the combined COAD prognostic model correlated significantly with the OS of COAD patients (Training cohort: likelihood ratio test = 45, p < 0.0001; Wald test = 41.93, p < 0.0001; score [log-rank] test = 50.34, p < 0.0001. Validation cohort: likelihood ratio test = 27.63, p < 0.0001; Wald test = 33.48, p < 0.0001; score [log-rank] test = 39.33, p < 0.0001). Using the median risk score of the training cohort as a cut-off value, we generated Kaplan-Meier curves and performed log-rank tests on the training cohort (Figure 5C) (p = 0.00011) and the validation cohort (Figure 5D) (p = 2e-05). Through these analyses, we sought to compare the OS of patients in the high-risk and low-risk groups and thus determine the predictive value of the combined COAD prognostic model based on six CpG sites. The risk scores for the training and validation cohorts are shown in Supplementary Tables 5 and 6. The survival rate of patients was significantly greater in the low-risk group than in the high-risk group. These results confirmed that our combined prognostic model based on six CpG sites could classify patients into high-risk and low-risk groups, demonstrating its clinical practicability.
To further evaluate the specificity of our combined COAD prognostic model in predicting survival, we used the AUC values obtained from time-dependent ROC analyses as categorical variables. In both the training cohort and the validation cohort, the combined COAD prognostic model precisely predicted the survival of COAD patients, with AUC values of 0.826 and 0.792, respectively (Figure 5E and 5F). We also performed univariate Cox regression analyses of the six individual CpG sites included in the COAD prognostic model (Supplementary Figure 1). The calculated AUC values indicated that the six individual CpG sites could also distinguish high-risk from low-risk patients; however, the predictive effect of any one CpG site was not as good as the predictive effect of the combined prognostic model using all six CpG sites. These results demonstrated that the six CpG sites may be potential prognostic biomarkers of COAD, but the combined COAD prognostic model based on six CpG sites is more valuable than the individual sites for clinical validation and prognostic evaluation.
Independence of the combined COAD prognostic model in OS prediction, and comparison of our prognostic model with other known prognostic models
To evaluate the stability and independence of our combined COAD prognostic model based on six CpG sites, we stratified patients according to clinicopathological characteristics such as age, gender, race, AJCC stage, examined lymph node count and lymphatic invasion. Remarkably, the Kaplan-Meier plots displayed significant extension of OS in the low-risk groups for all these characteristics in the 287 COAD samples from TCGA. Nevertheless, the combined COAD prognostic model predicted the survival of COAD patients more precisely than these factors, with an AUC value of 0.687 (Figure 6A–6C, Figure 7A–7C and Supplementary Figure 2). These results confirmed that the combined COAD prognostic model based on six CpG sites provided an excellent reference for different populations and was an independent predictor of patient survival.
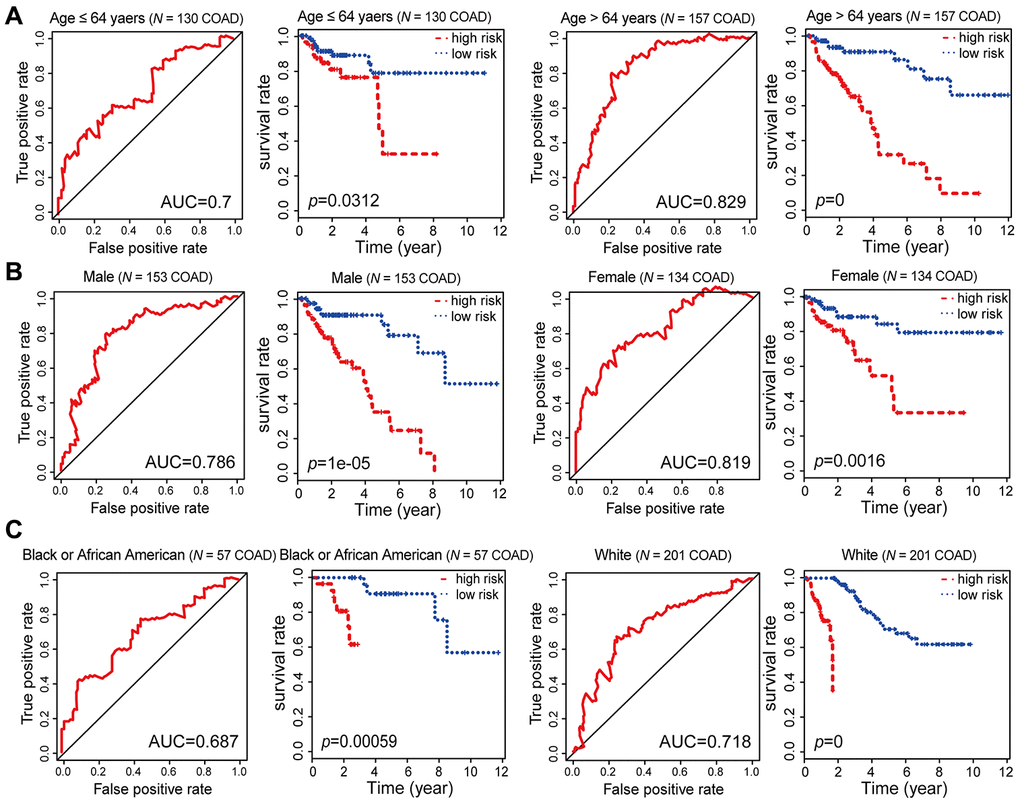
Figure 6. Kaplan-Meier and ROC analysis results based on age, gender and race. (A) Grouping of COAD patients according to their age at first diagnosis: ≤ 64 years (N = 130, 45.30%), > 64 years (N = 157, 54.70%). (B) Grouping of COAD patients according to gender: male (N = 153, 53.31%), female (N = 134, 46.69%). (C) Grouping of COAD patients according to race: black or African American (N = 57, 21.19%), white (N = 201, 74.72%).
![Kaplan-Meier and ROC analysis results based on stage, examined lymph node count and lymphatic invasion. (A) Grouping of COAD patients according to stage: early (stage I and II [N = 153, 53.31%]) and advanced (stage III and IV [N = 124, 43.21%]). (B) Grouping of COAD patients according to examined lymph node count: N = 42, 14.63%) and ≥ 12 (N = 226, 78.75%). (C) Grouping of COAD patients according to lymphatic invasion: lymphatic invasion (N = 76, 26.48%) and no lymphatic invasion (N = 175, 60.98%).](/article/103874/figure/f7/large)
Figure 7. Kaplan-Meier and ROC analysis results based on stage, examined lymph node count and lymphatic invasion. (A) Grouping of COAD patients according to stage: early (stage I and II [N = 153, 53.31%]) and advanced (stage III and IV [N = 124, 43.21%]). (B) Grouping of COAD patients according to examined lymph node count: < 12 (N = 42, 14.63%) and ≥ 12 (N = 226, 78.75%). (C) Grouping of COAD patients according to lymphatic invasion: lymphatic invasion (N = 76, 26.48%) and no lymphatic invasion (N = 175, 60.98%).
In recent years, DNA methylation biomarkers have been increasingly recognized as important prognostic predictors in COAD. Previously identified markers of COAD prognosis have included hypermethylation of FAM134B [27], higher expression of MMP-11 [28], abnormal methylation and expression of DIRAS1 [29], upregulation of the long non-coding RNA BLACAT1 (a cell cycle regulator) [30] and abnormal expression of 10 microRNAs (including hsa-mir-891a, hsa-mir-6854, etc.) [31]. Dai et al. [32] demonstrated that combined biomarkers of DNA methylation were more sensitive and specific than individual DNA methylation markers. To compare the survival prediction ability of our combined prognostic predictive model with those of previously reported biomarkers, we performed ROC analyses of the previous mRNA, long non-coding RNA and microRNA biomarkers in the validation cohort. Our combined COAD prognostic model based on six CpG sites had a much higher AUC value than the other prognostic biomarkers assayed by ROC analysis in the COAD validation cohort from TCGA (N = 144) (Figure 8A). These results suggested that our combined COAD prognostic model provided more reliable and sensitive predictions of OS than other biomarkers in COAD patients.
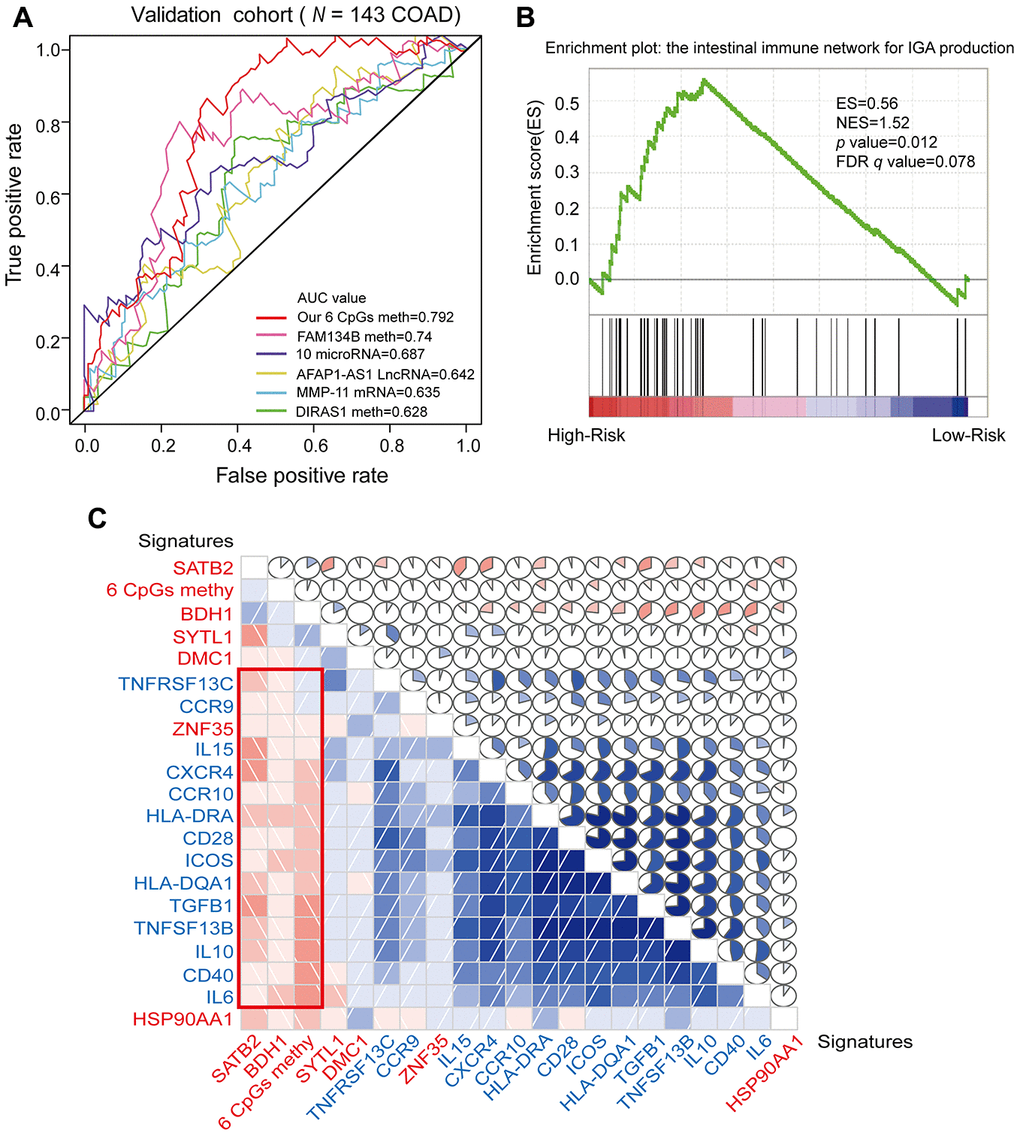
Figure 8. ROC analysis of different prognostic biomarkers and functional enrichment analysis of the corresponding genes. (A) ROC curve showing the sensitivity and specificity of our prognostic model and other known models in predicting the OS of patients in the validation cohort from TCGA. (B) COAD samples were divided into high-risk and low-risk groups, and the enrichment of IINIP pathway gene expression was analyzed using GSEA. ES, concentration fraction; NES, standardized ES; p-value, normalized p-value; FDR q-value, p-value corrected by the FDR method. (C) Correlation of the expression of the core enrichment genes from the IINIP pathway, the combined methylation level of our prognostic model and the expression of the genes corresponding to the individual CpG sites of the COAD prognostic biomarkers. The red signature represents the expression of the genes corresponding to the six CpG sites and the six-site combined methylation value; the blue signature represents the expression of the core enrichment genes in the IINIP pathway. Lower triangle: grids showing the correlation between two signatures, where blue indicates a positive correlation and red indicates a negative correlation. Upper triangle: circles represent the one-to-one correlation coefficients, differentiated by the fill area and intensity of shading. Blue indicates a positive correlation and red indicates a negative correlation.
Next, we performed a gene set enrichment analysis (GSEA) on the high- and low-risk groups that had been classified according to the median risk score. The pathways that correlated significantly with risk are illustrated in Figure 8B, Supplementary Figure 3 and Supplementary Table 7 (enrichment score [ES] > 0.4, |normalized enrichment score [NES]| > 1, p-value < 0.05 and FDR q-value < 0.25). We selected the intestinal immune network for IgA production (IINIP) for further analysis, since this pathway is known to be involved in COAD. The core enrichment genes of the IINIP pathway were obtained via GSEA (Supplementary Table 8). To determine whether the genes corresponding to the combined COAD prognostic biomarkers functioned in IINIP pathways, we conducted a one-to-one correlation analysis on the expression of the core enrichment genes from the IINIP pathway, the combined methylation level of our prognostic model and the expression of the genes corresponding to the individual CpG sites of the COAD prognostic biomarkers (Figure 8C and Supplementary Figure 4). As expected, the expression of BDH1 and SATB2 and the combined methylation level of our COAD prognostic model based on six CpG sites correlated significantly with the IINIP signaling pathway (p < 0.05). The above results indicated that our combined COAD prognostic model not only accurately predicted prognosis, but also included CpG sites that may directly or indirectly influence the IINIP pathway.